The Use of Artemisinin Compounds as Angiogenesis Inhibitors to Treat Cancer – Full Report
By Qigui Li, Peter Weina and Mark Hickman
1. Introduction
Angiogenesis takes place during development, and vascular remodeling is a controlled series of events leading to neovascularization, which supports changing tissue requirements. Blood vessels and stromal components are responsive to pro- and anti-angiogenic factors that allow vascular remodeling during development, wound healing and pregnancy. In pathological situations such as cancer, however, the same angiogenic signaling pathways are induced and exploited. Cancer angiogenesis is a requirement for the development and growth of solid tumors beyond 2–3 mm3 (Cao et al., 2011). Several angiogenic activators including members of the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) gene families and various inhibitors of angiogenesis have been described. In steady-state conditions, the balance between angiogenic activators and inhibitors results in very limited new blood vessel growth in the majority of tissues. The balance tilts in favor of the angiogenic stimulators, however, in a variety of proliferative processes. It is now generally accepted that angiogenesis is a rate-limiting process in tumor growth. Without new blood vessels to supply nutrients and dispose of catabolic products, tumor cells cannot sustain proliferation and thus are likely to remain dormant (Ferrara, 2010; Daniele et al., 2012).
Survival and proliferation of cancer depends on angiogenesis, which could be a target of cancer therapy. Angiogenesis is a complex physiological process. One example of this is found in the signaling pathways associated with the stimulus of various pro-angiogenic factors, VEGF and its receptors (VEGFR) which represents one of the best-validated signaling pathways in angiogenesis. A number of drugs approved by the FDA on market have been shown to inhibit anti-angiogenic pathway of VEGF. These agents include bevacizumab, a humanized anti-VEGF-A monoclonal antibody (Ferrara 2010), and two small molecule inhibitors targeting VEGFR2, sorafenib and sunitinib (Bergers and Hanahan 2008; Ellis and Hicklin 2008; Escudier et al., 2007; Motzer et al., 2007). Not all cancer patients, however, benefit from such anti-angiogenic therapies, and some that do benefit initially have been shown to become less responsive during the treatment as well as show some adverse effects over time (Bergers and Hanahan 2008; Chen and Cleck, 2009; Ellis and Hicklin 2008). Over the last few decades, numerous anti-angiogenic agents have been developed, and some of them have been tested in clinical settings. Angiogenesis includes a complex and multistep process, however, that has not been sufficiently elucidated. Hence, there is an urgent need to investigate the mechanisms that mediate resistance to anti-angiogenic agents. Recent advances have been made in identifying a number of novel alternate processes involved in angiogenesis. If these new findings of alternate mechanisms are confirmed, cancer therapy strategies may also be affected.
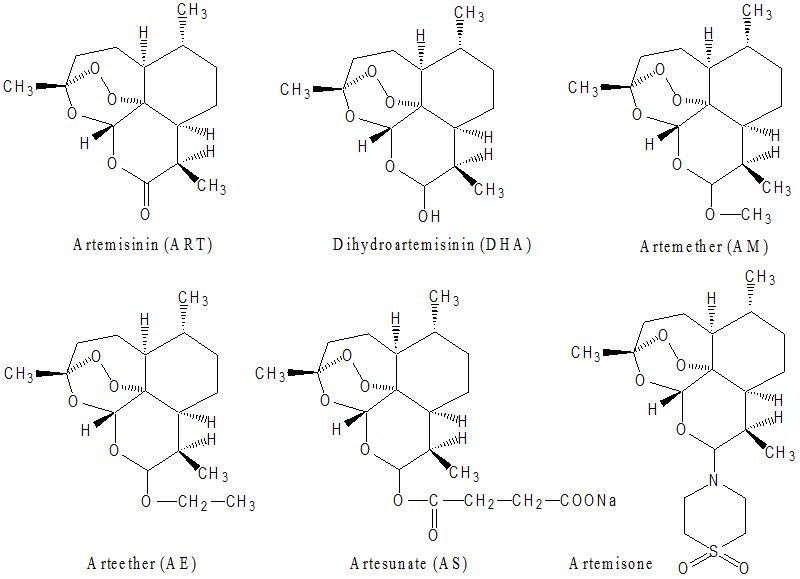
Artemisinin (ART) is a natural product of the plant Artemisia annua L. Reduction of ART yields the more active dihydroartemisinin (DHA), a compound which can be further converted to different derivatives, including, artesunate (AS) and artemether (AM), which are generally referred to as artemisinins (ARTs). ARTs are widely known for their potent antimalarial activity, but also been potential anti-cancer activity both in vitro and in vivo over the past few years. ARTs have inhibitory effects on cancer cell growth and also inhibit angiogenesis. Several studies have revealed that ART inhibits the growth of many transformed cell lines and has a selective cytotoxic effect. In one study, ART was shown to be more toxic to cancer than normal cells. In most of the systems, preloading of cancer cells with iron or iron-saturated holotransferrin triggers ART cytotoxicity with an increase in the activity of ARTs by 100-fold in some cell lines. It has been hypothesized that iron-activated ARTs induce damage by release of highly alkylating carbon-centered radicals and radical oxygen species (ROS). Radicals may play a role in the cell alterations reported in ARTs-treated cancer cells such as enhanced apoptosis, arrest of growth, inhibition of angiogenesis, and DNA damage. More studies have demonstrated that ART and its derivatives possess an anti-angiogenic activity (Li and Hickman, 2011).
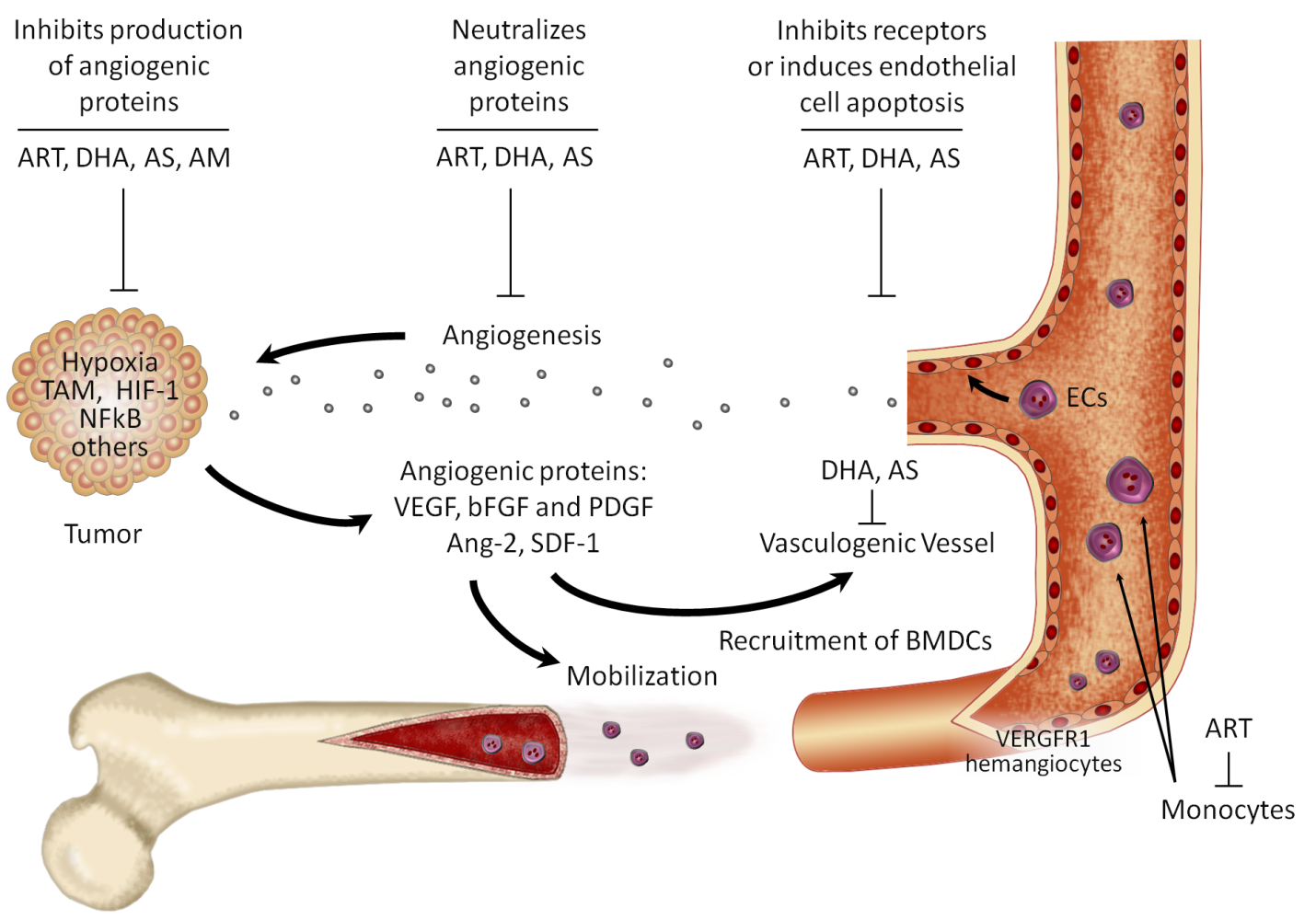
ARTs inhibit angiogenesis which is a vital process in metastasis. AS and DHA inhibit chorioallantoic membrane angiogenesis at low concentrations and decrease the levels of two major VEGF receptors on human umbilical vein endothelial cells (ECs). AS inhibits proliferation and differentiation of human microvascular dermal ECs in a dose-dependent manner and reduces Flt-1 and KDR/flk-1 expression. Conditioned media from K562 cells pretreated with AS and DHA inhibits VEGF expression and secretion in chronic myeloid leukemia K562 cells, leading to a decrease in genetic activity associated with angiogenesis. ARTs inhibit cell migration and concomitantly decrease the expression of matrix metalloproteinase proteins such as MMP2 and the avß3 integrins in human melanoma cells. ARTs also regulate the levels of urokinase plasminogen activator (u-PA), and the matrix metalloproteinases MMP2, MMP7 and MMP9 all of which are related to metastasis. Also, ARTs have been shown to increase production of reactive oxygen species and also inhibits the hypoxia induced production of a transcription factor, hypoxia inducible factor-1α (HIF1α). The HIF1α transcription factor increases tumor angiogenesis to support the survival of poorly nourished cancer cells. ARTs have shown pleiotropic effects through different experimental studies.
Definitely, ART compounds exhibit a wide spectrum of biological activities, including, for example, anti-angiogenic, anti-tumorigenic and even anti-viral, all of which are medically relevant. In particular, cancer angiogenesis plays a key role in the growth, invasion, and metastasis of cancers. After more than 30 years of intensive study, many agents, including novel candidate of ARTs, that target angiogenesis as cancer therapy and prevention of metastasis of existing tumors have been translated from the laboratory to the bedside. Therefore, ARTs-induced inhibition of angiogenesis could be a promising therapeutic strategy for treatment of cancer and prevention of metastasis. Various clinical trials using ARTs for anti-cancer therapy have been guided by the anti-angiogenesis research of ARTs that has been conducted anti-cancer. Since new and alternative angiogenesis mechanisms have been found, further research on the mechanism of anti-angiogenesis could lead us to understand more deeply the possibilities inherent in the development of ARTs for cancer therapy (Li and Hickman, 2011).
The new strategies for the development of ARTs for cancer therapy and metastasis prevention should include a plan for increasing their anti-angiogenic activity through a variety of approaches ranging from medicinal chemistry approaches to develop more potent ART-analogues to changes in formulation and/or dosing. The real potential and benefits of the ART drug class for cancer treatment and metastasis prevention remain yet to be discovered. Given the interest in using ARTs for cancer therapy, the door has been opened for challenging research in this area, which is likely to yield new cancer therapies that now do not exist. The aim of this chapter is to provide an overview of the recent advances and new development of this class of drugs as potential anti-angiogenic agents.
2. Activities of artemisinins (ARTs) as anti-cancer agents
Significant antitumor activity of ART and licensed semisynthetic its derivatives has been documented in vitro, in vivo and through clinical trials considerable research has been focused on the most active compounds, namely, artesunate (AS) and dihydroartemisinin (DHA).
2.1. ART and its derivatives
ART and its derivatives are lactonic sesquiterpenoid compounds first discovered in China. A crude extract of the wormwood plant Artemisia annua (qinghao) was first used as an antipyretic 2000 years ago. The antipyretic therapy dates back to the third century B.C. in the “Handbook of Prescriptions for Emergency Treatment” edited by Ge Hong (281-340 B.C.) where he recommended tea-brewed leaves of the wormwood plant to treat fever and chills. The specific effect of ART on the fever of malaria was reported in the 16th century in the “Compendium of Materia Medica” published by Li Shizen in 1596 cited Ge Hong’s prescription (Li and Weina, 2011). The active constituent of the extract was identified and purified in the 1970s, and named qinghaosu, or artemisinin (ART). Although ART proved effective in clinical trials in the 1980s, a number of semi-synthetic derivatives were developed to improve the drug’s pharmacological properties and antimalarial potency (Li et al., 2007). The structure of ART, which includes an endoperoxide bridge (C-O-O-C), is unique among antimalarial drugs. Semisynthetic ARTs are obtained from dihydroartemisinin (DHA), which is the reduced lactol derivative of ART, the main active metabolite of ARTs (Li et al., 1998). The first generation of semisynthetic ARTs includes the lipophilic arts, arteether (AE) and artemether (AM), while artesunate (AS) is the water soluble derivative (Li and Weina, 2011).
AS and its bioactive metabolite, DHA, have been the topic of considerable research attention in recent years for both anti-cancer and antimalarial indications. The key structural feature in all of the ART-related molecules that mediates their antimalarial activity, and some of their anti-cancer activities, is an endoperoxide bridge. The endoperoxides are a promising class of antimalarial drugs which may meet the dual challenges posed by drug-resistant parasites and the rapid progression of malarial illness. Of the available derivatives, AS has the most favorable pharmacological profile for use in ART-based combination therapy treatment of uncomplicated malaria and intravenous therapy of severe malaria (Li and Weina, 2010a). The effectiveness of AS has been mostly attributed to its rapid and extensive hydrolysis to DHA (Batty et al., 1998b; Davis et al., 2001; Li et al., 2009; Navaratnam et al., 2000).
Artemisone, a second-generation ART which is not metabolized to DHA, has shown improved pharmacokinetic properties including a longer half-life and lower toxicity (D’Alessandro et al., 2007; Schmuck et al., 2009) (Figure 1). Fully synthetic ART derivatives have also been designed by preserving the peroxide moiety which confers potent drug activity. These compounds are easily synthesized from simple starting materials; accordingly, these compounds are currently under intense development (Creek et al., 2008; Jefford 2007; Ramirez et al., 2009; Taylor et al., 2004). Hundreds of these compounds have been made; many resemble ART, but only one of these compounds, arteflene, has been taken beyond preclinical development (Radloff et al., 1996).
ART and its active derivatives have been widely used as antimalarial drugs for more than 30 years, and they have also been shown recently to be effective in killing cancer cells (Li et al., 2011). A number of studies demonstrated that ART and its bioactive derivatives exhibit potent anti-cancer effects in a variety of human cancer cell model systems. Recently, the anti-angiogenic activity of ARTs has been demonstrated, and these compounds have been shown to be potential anti-cancer agents (Crespo-Ortiz and Wei, 2012).
2.2. ARTs as first-line therapies for treatments of malaria
Global malaria control is being threatened on an unprecedented scale by rapidly growing resistance of P. falciparum to conventional monotherapies such as chloroquine, sulfadoxine-pyrimethamine (SP) and amodiaquine. Multi-drug resistant falciparum malaria is widely prevalent in South-East Asia and South America. Now Africa, the continent with highest burden of malaria is also being seriously affected by drug resistance. A significant advantage of ART and its derivatives in malaria treatment shows early evidence of cross-resistance to other antimalarial drugs. As a response to the rising tide of antimalarial drug resistance, WHO issued new Guideline for the Treatment of Malaria (WHO 2006; 2008) and recommends that treatment policies for falciparum malaria in all countries experiencing resistance to monotherapies should be combination therapies, preferably those containing an ART derivative.
2.2.1. WHO policies in malaria treatments
The pharmacological and clinical evaluations of ART group of drugs have been taken place for 30 years and four advantages have been evaluated.
- Rapid action and high efficacy against multi-drug resistant P. falciparum
- Evidence of ART drug resistance confirmed on the Cambodia-Thailand border
- Low toxicity (excellent safety profile)
- Gametocidal effect (prevents the transmission of malaria from person to person)
To treat uncomplicated malaria, the objective is to cure the infection. This is important as it will help prevent progression to severe disease and prevent additional morbidity associated with treatment failure. Cure of the infection translates to eradication of the parasite from the body. In treatment evaluations in all settings, emerging evidence indicates that it is necessary to follow patients for enough time to document a clinical cure. In assessing drug efficacy in high-transmission settings, temporary suppression of infection for 14 days has not been considered sufficient. The public health goal of treatment is to reduce transmission of the infection to others, i.e. to reduce the infectious reservoir. A secondary but equally important objective of treatment is to prevent the emergence and spread of resistance to antimalarials. Tolerability, the adverse effect profile and the speed of therapeutic response are also important considerations. A brief summary of the WHO policies (WHO, 2010) for treatment of uncomplicated falciparum malaria is listed below:
Artemisinin-based combination therapies (ACTs) are the treatment recommended by WHO in 2010 for all cases of uncomplicated falciparum malaria as first-line treatment including:
- artemether plus lumefantrine,
- artesunate plus amodiaquine,
- artesunate plus mefloquine,
- artesunate plus sulfadoxine-pyrimethamine,
- dihydroartemisinin plus piperaquine.
Second-line treatment:
- an effective alternative ACT (efficacy of ACTs depend on efficacy of the partner medicine, therefore it is possible to use two different ACTs as 1st and 2nd line options)
- quinine + tetracycline or doxycycline or clindamycin
Note: The ART derivatives (oral, rectal, or parenteral formulations) and partner medicines of ACTs are not recommended as monotherapy for uncomplicated malaria due to high rates of recrudescence associated with ART monotherapy.
To treat severe malaria, the primary objective of antimalarial treatment is to prevent death. Prevention of recrudescence and avoidance of minor adverse effects are secondary. In treating cerebral malaria, prevention of neurological deficit is also an important objective. In the treatment of severe malaria in pregnancy, saving the life of the mother is the primary objective. The following WHO policies are recommended for treatment of severe and complicated falciparum malaria as first-line treatment (WHO 2010):
Any of the following antimalarial medicines have been recommended by the WHO in 2010 for initial treatment.
- artesunate (i.v. or i.m.)
- artemether (i.m.)
- quinine (i.v. infusion or i.m. injection).
Follow-on treatment: once the patient recovers enough and can tolerate oral treatment, the following options can be used to complete treatment:
- full course of an ACT or
- quinine + clindamycin or doxycycline
Consistent with WHO recommendations (2006; 2010), malaria endemic countries which are experiencing resistance to currently used antimalarial drug monotherapies (chloroquine, sulphadoxine/pyrimethamine or amodiaquine) should change treatment policies to the highly effective ART-based combination treatments (ACTs).
2.2.2. ACT is a “policy standard” for first line malaria treatment
Antimalarial combination therapies can improve treatment efficacies of failing individual components and provide some protection for individual components against the development of higher levels of resistance. ACTs have been advocated as the best available option, and are the most commonly adopted regimen in countries changing antimalarial policy in the last decade. ACTs are most preferred for their enhancement of efficacy (Price 2000; White and Olliaro, 1998; White 1999a), lower malaria incidence and their potential to lower the rate at which resistance emerges and spreads (Nosten et al., 2000; White 1999b). Five ACTs recommended by a WHO Expert Consultative Group in 2010 include AM-lumefantrine (Coartem), AS-mefloquine (Artequin), AS-amodiaquine, and AS-sulfadoxine/pyrimethamine. Recently, WHO has endorsed ACTs as the “policy standard” for all malaria infections in areas where P. falciparum is the predominant infecting species (WHO 2006; 2007).
ARTs rapidly reduce parasitemia, but have poor efficacy as short course monotherapy. When used in combination with another agent, the rapid reduction in parasite numbers results in relatively few parasites being exposed to the second drug (to which significant resistance may already exist), theoretically preventing emergence of additional resistance mutations (White 2004). Furthermore, since ARTs themselves are not required to mediate final cure, there should also be little opportunity for ART resistance to develop. In addition, rapid reduction of the parasite burden in vivo by ACT drug combinations reduces the frequency of gametocyte generation, increases the rates of cure and may also reduce transmission of resistant parasites (Price, 2000). Most currently recommended drug combinations for falciparum malaria are variants of ACT where a rapidly acting ART compound is combined with a longer half-life drug of a different class. ARTs used include DHA, AS, AM and companion drugs include mefloquine, amodiaquine, sulfadoxine/pyrimethamine, lumefantrine, piperaquine, pyronaridine, and chlorproguanil/dapsone. The standard of care must be to cure malaria by killing the last parasite. Combination antimalarial treatment is vital not only to the successful treatment of individual patients but also for public health control of malaria.
ACTs continue to be the mainstay treatment of uncomplicated falciparum malaria. For the next 8–10 years, no alternative medicines to the ART derivatives able to offer similar high levels of therapeutic efficacy are expected to enter the market. For this reason, WHO has focused its efforts not only to increase access to quality ACTs, but also to contain the risk of development of falciparum resistance, associated with the large-scale use of oral monotherapies for treatment of uncomplicated malaria (WHO 2006; 2007).
In January 2006, WHO appealed to manufacturers to stop marketing oral ART monotherapies and instead to promote quality ACTs in line with WHO policy. This position has been widely disseminated via WHO Offices, WHO briefings to hospital staff and in regional and inter-country briefings to representatives of national health. Major procurement and funding agencies and international suppliers have accepted the WHO recommendation and agreed not to fund or procure oral ART monotherapies. In April 2006, the Global Malaria Programme of WHO provided a technical briefing to 25 pharmaceutical companies involved in the production and marketing of ART monotherapies. Out of these, 15 declared their willingness to stop marketing ART monotherapies over a short period of time, but 10 companies did not disclose their marketing plans for the future (meeting report available at: www.who.int/malaria/docs/ Meeting_briefing19April.pdf). In addition, some countries, like China and Pakistan, have been visited by WHO delegations to address multiple domestic manufacturers involved in this sector. The evolving position of manufacturers and of National Drug Regulatory Authorities (NDRA) in malaria endemic countries is monitored and displayed on the WHO Global Malaria Programme website front-page: http://malaria.who.int/.
In May 2007, the 60th World Health Assembly resolved to take strong action against oral monotherapies and approved the resolution WHA60.18, which:
- urges Member States to progressively cease the provision, in both the public and private sectors, of oral ART monotherapies, to promote the use of ART-combination therapies, and to implement policies that prohibit the production, marketing, distribution and the use of counterfeit antimalarial medicines;
- requests international organizations and financing bodies to adjust their policies so as progressively cease to fund the provision and distribution of oral ART monotherapies, and to join in campaigns to prohibit the production, marketing, distribution and use of counterfeit antimalarial medicines;
The above-mentioned benefits of ACTs make them an important tool for malaria treatment and control that has led to their increased use by 2010, most countries (89 countries), adopted ACTs as their first-line treatment of uncomplicated falciparum malaria. Only two countries adopted ACTs exclusively as second-line treatment (Bosman and Mendis, 2007).
2.3. Anti-cancer activities of ARTs
ART and its bioactive derivatives (AS, DHA, and AM) exhibit potent anti-cancer effects in a variety of human cancer cell model systems. The pleiotropic response in cancer cells to ART includes: 1) growth inhibition by cell cycle arrest, 2) apoptosis, 3) inhibition of angiogenesis, 4) disruption of cell migration, and 5) modulation of nuclear receptor responsiveness. These effects of ARTs result from perturbations of many cellular signaling pathways in vitro and in animal models. Considerable research has been focused on the most active ART compounds, namely, DHA and AS.
Molecular, cellular and physiological studies have demonstrated that, depending on the tissue type and experimental system, ART and its derivatives arrest cell growth, induce an apoptotic response, alter hormone responsive properties and/or inhibit angiogenesis of human cancer cells. The Developmental Therapeutics Program of the National Cancer Institute (NCI), USA, which analyzed the activity of AS on 55 human cancer cell lines (IC50 values shown between nano- to micro-molar range, depending on the cancer cell line), showed that AS displays inhibitory activity against leukemia, colon, melanoma, breast, ovarian, prostate, central nervous system (CNS), and renal cancer cells (Efferth et al., 2001; 2003; Efferth, 2006). DHA also has remarkable anti-neoplastic activity against pancreatic, leukemic, osteosarcoma, and lung cancer cells (Lu et al., 2009). Moreover, artemisone (second generation ART compound) has shown better activity than ART and considerable synergistic interactions with other anti-cancer agents (Gravett et al., 2010).
ART has been found to act either directly by inducing DNA damage (genotoxicity) or indirectly by interfering with a range of signaling pathways involved in several hallmarks of malignancy. Direct DNA damage is only described in specific systems, however, while indirect effects are more commonly noted in the literature. In pancreatic cells (Panc-1), artesunate was shown to cause DNA fragmentation and membrane damage. Interestingly, low doses of artesunate were associated with oncosis-like cell death, whereas higher concentrations were shown to induce apoptosis (Du et al., 2010). The extent and type of cellular damage seems to depend on the phenotype and the origin of cell line, and it may also vary in a time- and dose-dependent manner (Crespo-Ortiz and Wei, 2012). Notably, higher sensitivity to AS was observed in rapidly growing cell lines when compared with slow growing cancer cells (Efferth et al., 2003).
Moreover, the highly stable ARTs and ART-derived trioxane dimers were shown to inhibit growth and selectively kill several human cancer cell lines without inducing cytotoxic effects on normal neighboring cells. One proposed mechanism by which ART targets cancer cells involves cleavage of the endoperoxide bridge by the relatively high concentrations of iron in cancer cells, resulting in iron depletion in those cells coupled with generation of free radicals such as reactive oxygen species (ROS) capable of inducing subsequent oxidative damage. This mechanism resembles the known mechanism of action of ART in malarial parasites. In addition to possessing higher iron influx via transferrin receptors, cancer cells are also sensitive to oxygen radicals because of a relative deficiency in antioxidant enzymes. A significant positive correlation can be made between AS sensitivity and transferrin receptor levels as well as between AS sensitivity and expression of ATP binding cassette transporters (Efferth, 2006).
Expression profiling of several classes of tumor cells has shown that ART treatment caused selective expression changes of many oncogenes and tumor suppressor genes than genes responsible for iron metabolism, which suggests that the anti-cancer properties of ARTs cannot be explained simply by the global toxic effects of oxidative damage. Alternatively, DHA, AS, and AM may well be to modulating genes and proteins coordinating growth signals, apoptosis, proliferation capacity, angiogenesis and tissue invasion, and metastasis. A complex network of interactions through different pathways may enhance the anti-cancer effect of these endoperoxide drugs leading to cancer control and cell death (Crespo-Ortiz and Wei, 2012).
ARTs have also been observed to attenuate multidrug resistance in cancer patients, an effect due in part to the inhibition of glutathione S-transferase activity. ART and its bioactive derivatives elicit their anti-cancer effects by concurrently activating, inhibiting and/or attenuating multiple complementary cell signaling pathways, which have been described in a variety of human cancer cell systems as well as in athymic mouse xenograft models. The ART compounds exert common as well as distinct cellular effects depending on the phenotype and tissue origin of the human cancer cells tested. (Firestone and Sundar 2009)
2.4. Anti-cancer mechanism of ART and its derivatives
The anti-cancer potential of ARTs has been demonstrated in various cancer cells including those of leukemia and other cancer cells of breast, ovary, liver, lung, pancreas and colon (Tan et al., 2011).The mechanisms of action of ARTs in cancer cells are associated with: 1) anti-angiogenic effects, 2) induction of apoptosis, 3) oxidative stress response, 4) oncogenes and tumor suppressor genes, and 5) multidrug resistance (Figure 2) (Efferth 2006; 2007).
2.4.1. Anti-cancer mechanism of ARTs based on antimalarial actions
The endoperoxide moiety of ART has been shown to be pharmacologically important and responsible for antimalarial activity against the malaria parasites. The potent anti-cancer action of ARTs can be also attributed to the endoperoxide bond. In most of the in vitro cancer cell lines tested, preloading of cancer cells with iron or iron-saturated holotransferrin triggers ART cytotoxicity with an increase in ARTs activity up to 100-fold against some cell lines. It has been hypothesized that iron-activated ARTs induce damage by release of highly alkylating carbon-centered radicals and ROS. Radicals may play a role in the cell alterations reported in ARTs-treated cancer cells such as enhanced apoptosis, arrest of growth, inhibition of angiogenesis, and DNA damage. Microarray analyses found that the action of ARTs seems to be modulated by the expression of oxidative stress enzymes including catalase, thioredoxin reductase, superoxide dismutase and the glutathione S-transferase family. ARTs-sensitive cells demonstrate down-regulated oxidation enzymes whereas over-expression of these enzymes renders cancer cells less sensitive to chemotherapeutic agents. The antineoplastic toxicity of ARTs appears to be also modulated by calcium metabolism, endoplasmic reticulum (ER) stress, and the expression of the translationally controlled tumor protein, TCTP, a calcium binding protein which has been also postulated as a parasite target. Although the expression of the TCTP gen, tctp, was initially correlated with cancer cell response to ARTs, a functional role for TCTP in the action of ARTs has yet to be found. As for malaria parasites, the role of sarcoendoplasmic Ca2+ ATPase (SERCA) as a target of ARTs in cancer cells has also been explored (Crespo-Ortiz and Wei, 2012).
Expression profiling of several classes of tumor cells has shown that ART treatment causes selective expression changes of many more oncogenes and tumor suppressor genes than genes responsible for iron metabolism, which suggests that the anti-cancer properties of ART cannot be explained simply by the global toxic effects of oxidative damage. ART has also been observed to attenuate multidrug resistance in cancer patients, an effect due in part to the inhibition of glutathione S-transferase activity. ART and its bioactive derivatives elicit their anti-cancer effects by concurrently activating, inhibiting and/or attenuating multiple complementary cell signaling pathways, which have been described in a variety of human cancer cell systems as well as in athymic mouse xenograft models. The ART compounds exert common as well as distinct cellular effects depending on the phenotype and tissue origin of the human cancer cells tested. (Firestone and Sundar, 2009).
2.4.2. Potential general mechanisms of ART and its derivatives
Studies have identified potential general anti-cancer mechanisms of anti-cancer ARTs such as normalization of the upregulated Wnt/β-catenin pathway in colorectal cancer. Other pathways for anti-cancer activity include inhibition of enhanced angiogenesis associated with tumors. ARTs have been shown to inhibit proliferation, migration and tube formation of human umbilical vein endothelial cells (HUVEC), inhibit VEGF binding to surface receptors on HUVEC and reduce expression of VEGF receptors Flt-1 and KDR/flk-1 on HUVECs. In cancer cells, artemisinins reduce expression of the VEGF receptor KDR/flk-1 in tumor and endothelial cells and slow the growth of human ovarian cancer HO-8910 xenografts in nude mice. HUVEC apoptosis by artesunate is associated with downregulation of Bcl-2 (B-cell leukemia/lymphoma 2) and upregulation of BAX (Bcl-2-associated X protein). In addition, mRNA expression of 30 out of 90 angiogenesis-related genes correlated significantly with the cellular response to ARTs, supporting the hypothesis that ARTs exert their anti-tumor effects by inhibition of tumor angiogenesis (Krishna et al., 2008).
2.4.3. Anti-angiogenesis of ARTs including Anti-proliferation
In the process of angiogenesis, the formation of new blood vessels from pre-existing ones is essential for the supply of tumors with oxygen and nutrients. If cancers reach a size for which diffusion alone cannot supply enough oxygen and nutrients angiogenesis is promoted by numerous pro-angiogenic or anti-angiogenic factors. The anti-angiogenic activities of ARTs were shown using various models of angiogenesis, namely, proliferation, migration and tube formation of endothelial cells. As a consequence, inhibitors of angiogenesis were considered as interesting possibilities for cancer therapy. As shown by several groups around the world, ART and its derivatives inhibit angiogenesis, and a detailed description of the ART-induced anti-angiogenic mechanisms will be described in Section 3.
2.4.4. Induction of apoptosis
ARTs induce cell cycle arrest in various cell types (Efferth, 2006). For example, DHA and AS effectively mediate G1 phase arrest in HepG2 and Hep3B cells (Hou et al., 2008), and DHA treatment has been shown to reduce cell numbers of HCT116 colon cancer cells in S phase (Lu et al., 2011). Interestingly, DHA treatment has also been shown to trigger G2 phase arrest in OVCA-420 ovarian cancer cells (Jiao et al., 2007). Thus, ART-mediated cell cycle arrest is possibly cell type dependent. ARTs have also been shown to induce apoptotic cell death in a number of cell types, in which the mitochondrial-mediated apoptotic pathway plays a decisive role (Lu et al., 2011). For instance, DHA has been shown to enhance Bax and reduces Bcl-2 expression in cancer cells (Hou et al., 2008; Chen et al., 2009). DHA-induced apoptosis is abrogated by the loss of Bak and is largely reduced in cells with siRNA-mediated down-regulation of Bak or NOXA (Handrick et al., 2010). DHA has been shown to activate caspase-8, however, which is related to the death receptor-mediated apoptotic pathway in HL-60 cells (Liu et al., 2008). DHA has also been shown to enhance Fas expression and activates caspase-8 in ovarian cancer cells (Chen et al., 2009). In addition, DHA enhances death receptor 5 and activates both mitochondrial- and death receptor-mediated apoptotic pathways in prostate cancer cells (He et al., 2010). ARTs-induced apoptosis in cancer cells may involve p38 MAPK, however, rather than p53 (Hou et al., 2008; Lu et al., 2008).
Since most anti-cancer drugs kill tumor cells by the induction of apoptosis, the same may be true for ART and its derivatives. AS was first shown to promote apoptosis in tumor cells (Efferth et al., 1996). This has been subsequently confirmed by other groups (Li et al., 2001; Sadava et al., 2002; Singh and Lai, 2004; Wang et al., 2002; Yamachika et al., 2004). By microarray and hierarchical cluster analyses, several apoptosis-regulating genes were identified, whose mRNA expression correlated significantly with the IC50 values for AS in the NCI cancer cell lines (Efferth et al., 2003).
2.4.5. Oxidative stress response
ART is first activated in malaria parasites by intra-parasitic heme-iron, which catalyzes the cleavage of the endoperoxide bond. The Plasmodium trophozoites and schizonts live within red blood cells, where hemoglobin serves as an amino acid source. It is taken up by the parasites into food vacuoles, where enzymatic degradation takes place (Semenov et al., 1998; Shenai et al., 2000). The release of heme-iron during hemoglobin digestion facilitates the cleavage of the endoperoxide moiety by a Fe (II) Fenton reaction. Breaking the endoperoxide bridge of ART results in the generation of reactive oxygen species, such as hydroxyl radicals and superoxide anions, which damage the food vacuole membranes and leads to subsequent auto-digestion (Krishna et al., 2004; O’Neill and Posner, 2004). In addition, the heme iron (II)-mediated decomposition of ART leads to the generation of carbon-centered radical species (Butler et al., 1998). The cleavage of the endoperoxide bond of ART and its derivatives also leads to the alkylation of heme and some Plasmodium-specific proteins, including the Plasmodium falciparum translationally controlled tumor protein (TCTP) and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) ortholog of Plasmodium falciparum (Eckstein-Ludwig et al., 2003). Recent observations indicate, however, that heme iron (II) and oxidative stress are not the only mechanisms of ART’s anti-malarial activity (Parapini et al., 2004).
By comparing the baseline antioxidant mRNA gene expression in the NCI cell line panel with the IC50 values for AS, oxidative stress was found to play a role in the anti-tumor activity of AS (Efferth, 2006). The expression of thioredoxin reductase and catalase correlated significantly with the IC50 values for AS against the tumor cell lines in the NCI panel. As tumor cells contain much less iron than erythrocytes, but more than other normal tissues (Shterman et al., 1991), the question arises as to whether iron may be critical for ART’s activity against tumor cells (Payne, 2003). The growth of tumors in rats was significantly retarded by daily oral administration of ferrous sulfate followed by dihydroartemisinin, while treatment with each drug applied alone had no effect (Moore et al., 1995). Cellular iron uptake and internalization are mediated by binding of transferrin-iron complexes to the transferrin receptor (CD71) expressed on the cell surface membrane which leads to subsequent iron endocytosis. CD71 is normally expressed in the basal epidermis, endocrine pancreas, hepatocytes, Kupfer cells, testis, and pituitary, while most other tissues are CD71-negative. In contrast, CD71 is highly expressed in proliferating and malignant cells (Sutherland et al., 1981) and it is widely distributed among clinical tumors (Gatter et al., 1983).
Interestingly, exposure of ART and its derivatives produces no or only marginal cytotoxicity to non-tumor cells. Human breast cells do not respond to treatment with transferrin plus DHA, while the growth of breast cancer cells is significantly inhibited (Singh and Lai, 2001). Similarly, ART tagged to transferrin has been shown to be more cytotoxic to MOLT-4 leukemia cells than to normal lymphocytes (Lai et al., 2005).
2.4.6. Oncogenes and tumor suppressor genes
Oncogenes and tumor suppressor genes frequently affect downstream processes in tumor cells. The expression of several oncogenes and tumor suppressor genes has been shown to correlate with response to artesunate, including the epidermal growth factor receptor (EGFR), the tumor growth factor ß (TGFB), FBJ murine osteosarcoma viral oncogene homologue B (FOSB), FOS-like antigen-2 (FOSL2), the multiple endocrine neoplasia 1 gene (MEN1), v-myb avian myeloblastosis viral oncogene homolog (MYB), v-myc avian myelocytomatosis viral oncogene homolog
(MYC), c-src tyrosine kinase (CSK), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), the RAS oncogene family members ARHC, ARHE, RAB2 and RAN, the breast cancer susceptibility gene 2 (BRCA2), and others (Efferth et al., 2003).
The epidermal growth factor receptor (EGFR) represents an exquisite target for therapeutic interventions, and molecular approaches to study the expression of the EGFR gene have yielded some very interesting findings. Glioblastoma cells transfected with a deletion-activated EGFR cDNA were more resistant to AS than the control cells which agrees well with microarray gene expression data (Efferth et al., 2003). In addition to playing a role in drug resistance, the activation of EGFR-coupled signaling routes drives mitogenic and other cancer-promoting processes, e.g. proliferation, angiogenesis, and inhibition of apoptosis (Efferth 2006). In addition, combination treatment of the EGFR tyrosine kinase inhibitor, OSI-774, plus AS was investigated and synergistic effects were found in glioblastoma cells transfected with a deletion-activated EGFR cDNA, and additive effects were shown to occur in cells transfected with wild-type EGFR (Efferth et al., 2004a). A profile of chromosomal gains and losses was determined by comparative genomic hybridization in nine non-transfected glioblastoma cell lines, and this profile correlated well with the IC50 values determined after treatment of the same glioblastoma cell lines with the combination treatment of AS and OSI-774. Genes located at genomic loci correlating to cellular response to AS and OSI-774 may serve as candidate genes to determine drug sensitivity and resistance (Efferth 2007).
By screening a panel of isogenic Saccaromyces cerevisiae strains with defined genetic mutations in DNA repair, DNA checkpoint, and cell proliferation genes, one yeast strain with a defective mitosis-regulating BUB3 gene showed increased sensitivity to AS treatment. Another strain with a defective proliferation-regulating CLN2 gene showed increased AS resistance over the wild-type strain. None of the other DNA repair or DNA check-point deficient isogenic strains were different from wild-type yeast (Efferth et al., 2001). The conditional expression of the CDC25A gene by a tetracycline repressor expression vector (tet-off system) has been shown to increase cellular sensitivity to AS treatment (Efferth et al., 2003). CDC25A is a key regulator of the cell cycle, which drives cells from the G1 phase into S phase. AS has been shown to down-regulate the expression of the CDC25A protein which supports the hypothesis that AS interferes with cell cycle regulation (Efferth et al., 2003).
The IC50 values for artesunate were correlated with the constitutive mRNA expression levels measured by microarray hybridization. Scientists selected expression data of 559 genes deposited in the NCI’s database (http://dtp.nci.nih.gov). The mRNA expression has been determined as reported. These genes belong to different categories of biological functions (63 apoptosis-regulating genes, 113 proliferation associated genes, 140 anti-oxidative stress response genes, 90 angiogenesis-regulating genes, 123 oncogenes and tumor suppressor genes). For example, p53, the ‘‘guardian of the genome’’, is a transcription factor that can bind to promoter regions of hundreds of genes where it either activates or suppresses gene expression. Thereby, p53 serves as a tumor suppressor by inducing cell cycle arrest, apoptosis, senescence and DNA repair. In normal cells, p53 is frequently undetectable due to fast ubiquitination by mdm-2 and subsequent proteasomal degradation. However, upon DNA damage and several other stresses, including drug stress, the amount of p53 is increased due to disruption of its degradation. Artesunate could inhibit HSCs proliferation in vitro through increase the expression of p53 (Efferth et al., 2006; Hou et al., 2008; Lu et al., 2008).
2.4.7. Multidrug resistance
A prominent feature of ART and its derivatives in malaria treatment shows early signs of cross-resistance to other antimalarial drugs. ARTs are therefore very valuable for the treatment of otherwise unresponsive, multidrug-resistant malaria parasites (Li and Weina 2011). Therefore, it is reasonable to ask whether ARTs are involved in the multidrug-resistance phenotypes observed in tumor cells. A comparison of the microarray-based mRNA expression of the multidrug resistance-conferring ABCB1 gene (MDR1; P-glycoprotein) was conducted with the IC50 values determined for tumor cells treated with AS and dihydroartemisinyl ester stereoisomer 1, but no significant relationships were observed.
Similarly, the flow cytometric measurement of the fluorescent probe rhodamine 123, which represents a functional assay for P-glycoprotein, did not reveal significant correlations, and similar results were obtained with other ARTs. As a control, we used the established anti-tumor drug docetaxel (taxotere), which is a known substrate of MDR1 (Shirakawa et al., 1999). The IC50 values determined for cells treated with docetaxel correlated both with rhodamine 123 efflux and MDR1 mRNA expression. To validate these results obtained by correlation analyses, cell lines over-expressing MDR1/P-glycoprotein as well as other drug resistance-conferring genes were used. AS was shown similarly active towards drug-sensitive and multidrug resistant cell lines (Efferth et al., 2002; 2003). Likewise, methotrexate-resistant CEM/MTX1500LV cells with an amplification of the dihydrofolate reductase (DHFR) gene and hydroxyurea-resistant CEM/HUR90 cells with over-expression of ribonucleotide reductase (RRPM2) were not cross-resistant to AS. In addition, other research has shown that ART increased the tissue permeability for standard cytostatic drugs. i.e. doxorubicin in mouse embryonic stem cell-derived embryoid bodies (Wartenberg et al., 2003).
3. Anti-cancer effect of ARTs via an anti-angiogenic activity
In the process of angiogenesis, the formation of new blood vessels from pre-existing ones is essential for the supply of tumors with oxygen and nutrients and for the spread of metastatic cells throughout the body. Normal angiogenesis is strictly controlled by some transient, typical physiological processes such as reproduction, development, wound healing; continued angiogenesis is also a characteristic of pathological alteration such as neoplasia. Neoplasia is an angiogenesis-dependent disease, and the growth of tumors, intravasation and metastases require angiogenesis. In human and experimental cancers, new vessels are required for increased delivery of nutrients and are a target for invading tumor cells, and there is a large body of evidence to support a key role for angiogenesis in disease progression. The growth, invasion and metastasis of tumors have been shown to be dependent on angiogenesis. A summary of the anti-angiogenic effects of ARTs is shown in Table 1.
| Artemisinins | Effects/Mechanism | References |
| Artesunate (AS) | 1) Induction of apoptosis in KS-IMM cells 2) Reduced F1t-1 and KDR/flk-1 expressions 3) Lowered VEGF and KDR/flk-1 expression 4) inhibited the proliferation of HUVEC 5) Inhibited HUVEC and VEGF expression 6) Suppress angiogenic ability & Decreased VEGF 7) Decreased HIF-1α levels 8) Decreased VEGF and Ang-1 secretion 9) Decreased the secretion of VEGF and IL-8 10) Either increased cytotoxicity or cytostasis | Dell’Eva et al., 2004 Huan-huan et al., 2004 Chen et al., 2004a Chen et al., 2004b Chen et al., 2004c Zhou et al., 2007 Zhou et al., 2007 Chen et al., 2010a He et al., 2011 Liu et al., 2011 |
| Dihydro-artemisinin (DHA) | 1) DHA was more effective than AS 2) Reduced VEGF binding to its receptors 3) Induced K562 cells apoptosis, inhibited VEGF 4) Reduced VEGF secretion by RPMI8226 cells 5) Attenuated the levels of VEGFR-3/Flt-4. 6) Decreased KDR levels and NF-kB DNA binding 7) Inhibition of PKCalpha/Raf/MAPKs 8) Decreased VEGF receptor KDR/flk-1 9) Inhibited the expression of several MMPs 10) DHA inactivates NF-kappaB and potentiates 11) Down-regulated VEGF 12) Inducted iron-dependent endoplasmic reticulum stress 13) DHA inhibits formation of HUVECs, MMP9 | Chen et al., 2003 Chen et al., 2004a Lee et al., 2006 Wu et al., 2006 Wang et al., 2007 Chen et al., 2010b Hwang et al., 2010 Zhou et al., 2010 Rasheed et al., 2010 Wang et al., 2010 Aung et al., 2011 Lu et al., 2011 Wang et al., 2011 |
| Artemisinin (ART) | 1) Decreased VEGF-A transcription 2) Decreased MMP2, MMP9 and BMP1 levels 3) Decreased VEGF-C, IL-1 β-induced p38 4) Decreased αvβ3 transcription | Anfosso et al., 2006 Anfosso et al., 2006 Wang et al., 2008 Buommino et al., 2009 |
| 2nd Artemisinin artemisone | less anti-angiogenic effect than DHA in all the experimental models | D’Alessandro et al., 2007 |
| Artemisinin-like compounds (ART-like) | 1) Active against solid tumor-derived cell lines and good correlation with other ARTs 2) More active in vitro and in vivo than the commonly used AS | Galal et al., 2009 Soomro et al., 2011 |
| Thioacetal ARTs | inhibitiory activity upon HUVEC | Oh et al., 2003 |
| ART-glycolipid hybrids | Showed potent in vivo anti-angiogenic activity on CAM | Ricci et al., 2010 |
Table 1.
Anti-angiogenic effects of ART and its derivatives
VEGF = vascular endothelial growth factor; HIF = hypoxia-inducible factor; NF-kB = nuclear factor of kappa light polypeptide gene enhancer in B cells 1; KDR = kinase insert domain protein recepto; MMP = matrix metalloproteinase; BMP = bone morphogenic protein; αvβ3 = Transmembrane heterodimeric protein expressed on sprouting endothelial cells; HUVEC = human umbilical vein endothelial cells. CAM = chorioallantoic membrane
3.1. Anti-angiogenic effects of ARTs
3.1.1. In vitro anti-angiogenic effects of ART and its derivatives
While most of the research on the anti-cancer activities of ARTs has been performed with cell lines in vitro, there are a few reports in the literature showing activity in vivo against xenograft tumors, e.g., breast tumors, ovarian cancer, Kaposi sarcoma, fibrosarcoma, or liver cancer. The in vitro data in the literature supports the hypothesis that ART and its derivatives kill or inhibit the growth of many types of cancer cell lines, including drug-resistant cell lines, suggesting that ART could become the basis of a new class of anti-cancer drugs. In addition, the co-administration of holotransferrin and other iron sources with ARTs has been shown to increase the potency of ARTs in killing cancer cells.
Artemisinin (ART)
ARTs are antimalarial agents, but also reveal profound antitumor activity in vitro and in vivo. Ina microarray study of cancer cells treated at the 50% inhibition concentration with eight ARTs, (ART, AS, arteether, artemisetene, arteanuine B, dihydroartemisinylester stereoisomers 1 and 2) the mRNA expression data of 89 known angiogenesis-related genes was obtained and correlated against the sensitivity of these tumor cells to ARTs treatment. The constitutive expression of 30 genes correlated significantly with the cellular response to ARTs. The finding cell sensitivity and resistance of tumor cells could be predicted by the mRNA expression of angiogenesis related genes supports the hypothesis that ARTs reveal their antitumor effects at least, in part, by inhibition of tumor angiogenesis. As many chemo-preventive drugs exert anti-angiogenic features, ARTs might also be chemo-preventive in addition to their cytotoxic effects (Anfosso et al., 2006).
A recent study demonstrated that ART-induced cell growth arrest in A375M malignant melanoma tumor cells also affected the viability of A375P cutaneous melanoma tumor cells with both cytotoxic and growth inhibitory effects, while ART was not effective in inhibiting the growth of other tumor cell lines (MCF7 and MKN). In addition, ART treatment affected the migratory ability of A375M cells by reducing metalloproteinase 2 (MMP-2) productions and down-regulating αvβ3 integrin expression. These findings support the hypothesis that ART may serve as a chemotherapeutic agent for melanoma treatment (Buommino et al., 2009). Furthermore, IL-1beta-induced p38 mitogen-activated protein kinase (MAPK) activation and upregulation of VEGF-C mRNA, and VEGF-C receptor protein levels in LLC cells were also suppressed by ART or by the p38 MAPK inhibitor SB-203580, suggesting that p38 MAPK could serve as a mediator of pro-inflammatory cytokine-induced VEGF-C expression. These data support the hypothesis that ART may be useful for the prevention of lymph node metastasis by downregulating VEGF-C and reducing tumor lymphangiogenesis (Wang et al., 2008).
Dihydroartemisinin (DHA)
DHA and AS have been shown to be remarkable inhibitors of tumor cell growth and suppression of angiogenesis in vitro. The anti-cancer activity of ARTs has been demonstrated by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) growth inhibition assay of four human cancer cell lines, cervical cancer HeLa, uterus chorion cancer JAR, embryo transversal cancer RD and ovarian cancer HO-8910 treated with DHA and AS. IC50 values obtained through this MTT growth inhibition assay demonstrated that DHA was more effective at inhibiting cancer cell lines than AS. The anti-angiogenic activities of DHA and AS were tested on in vitro models of angiogenesis by assessing the proliferation, migration and tube formation of human umbilical vein endothelial (HUVE) cells. The results showed that DHA and AS significantly inhibited angiogenesis in a dose-dependent manner. These results also showed that DHA was more effective than ART in inhibiting angiogenesis (Chen et al., 2003).
The effect of DHA on human multiple myeloma-induced angiogenesis under hypoxia and elucidated its mechanism of action has been performed. An in vivo chicken chorioallantoic membrane model was used to examine the effect of DHA on multiple myeloma-induced angiogenesis. Compared with conditioned medium of control, conditioned medium from human multiple myeloma RPMI8226 cells pretreated with 3 µM DHA in hypoxia was observed to reduce microvessel growth on chicken chorioallantoic membranes by approximately 28.6% (P < 0.05). The level of VEGF in conditioned medium was determined by enzyme-linked immunosorbent assay. The results confirmed that 3 µM DHA could significantly decrease VEGF secretion by RPMI8226 cells (P < 0.05), which correlated well with the reduction of multiple myeloma-induced angiogenesis on chicken chorioallantoic membranes. Western blot and reverse transcription-PCR results revealed that DHA downregulated the expression of VEGF in RPMI8226 cells in hypoxia. Therefore, DHA possesses potential as an antiangiogenic drug in multiple myeloma therapy and thereby may improve patient outcome (Wu et al., 2006).
The effect of DHA on VEGF expression and apoptosis in chronic myeloid leukemia (CML) K562 cells was assessed. The results demonstrated that in addition to its anti-proliferation effect on CML cells, DHA was also found to induce K562 cells apoptosis. The percentage of apoptotic cells was increased to 6.9 and 15.8% after being treated with 5 and 10 µM DHA for 48 h, respectively (P < 0.001). All these experiments suggested that DHA could inhibit the VEGF expression and secretion effectively in K562 cells, even at a lower concentration (2 µM, P < 0.05). Moreover, we further assessed the stimulating angiogenic activity of CM from K562 cells on CAM model. Also, the angiogenic activity was decreased in response to the CM from K562 cells pretreated with DHA in a dose-dependent manner. Taken together, these results from our study together with its known low toxicity make it possible that DHA might present potential anti-leukemia effect as a treatment for CML therapy, or as an adjunct to standard chemotherapeutic regimens (Lee et al., 2006)
DHA was found to have a potent ability in influencing lymphatic endothelial cells (LECs) behavior. DHA also exerted a significant inhibitory effect on migration and tube-like formation of LECs in a dose-dependent manner. Quantitative RT-PCR further showed that DHA remarkably downregulated the expression of antiapoptotic bcl-2 mRNA, but upregulated that of the proapoptotic gene bax mRNA. In addition, DHA could strongly attenuate the mRNA and protein levels of VEGFR-3/Flt-4. In summary, these findings indicate that DHA may be useful as a potential lymphangiogenesis inhibitor under induction of cell apoptosis, inhibition of the migration, and formation of tube-like structures in LECs (Wang et al., 2007). In addition, to investigate the effects of DHA on cell cycle progression and NF-kappaB activity in pancreatic cancer cells, the cell cycle progression was determined. The translocation and DNA-binding activity of NF-kappaB were inhibited in DHA-treated cells in a dose-dependent manner, indicated the inactivation effects of DHA in pancreatic cancer cells. Study shows that DHA induces cell cycle arrest and apoptosis in pancreatic cancer cells, and this effect might be due to inhibition of NF-kappaB signaling (Chen et al., 2010b).
One study showed that DHA is an effective anti-metastatic agent that functions by down-regulating the MMP-9 gene which is associated with metastasis. 1) DHA was shown to reduce phorbol myristate acetate (PMA)-induced activation of MMP-9 and MMP-2 and further inhibited cell invasion and migration. 2) DHA was also shown to suppress the PMA-enhanced expression of the levels of MMP-9 protein and mRNA, and enhanced transcriptional activity of the MM-9 gene through suppression of NF-kappaB and activation of AP-1 without changing the level of tissue inhibition of metalloproteinase (TIMP)-1. 3) DHA has been shown to reduce PMA-enhanced MMP-2 expression by suppressing membrane-type 1 MMP (MT1-MMP), but was not shown to t alter TIMP-2 levels. 4) DHA was shown to inhibit PMA-induced NF-kappaB and c-Jun nuclear translocation, which are upstream of PMA-induced MMP-9 expression which enhances metastasis. 5) DHA strongly repressed the PMA-induced phosphorylation of Raf/ERK and JNK, which are dependent on the PKC alpha pathway. In summary, this study demonstrated that the anti-invasive effects of DHA may occur through inhibition of PKC alpha/Raf/ERK and JNK phosphorylation and reduction of NF-kappaB and AP-1 activation, leading to down-regulation of MMP-9 expression. (Hwang et al., 2010)
Wang et al. demonstrated that DHA enhances gemcitabine-induced growth inhibition and apoptosis in both BxPC-3 and PANC-1 cell lines in vitro. The effect is at least partially due to the DHA-driven deactivation of gemcitabine-induced NF-kappaB activation, which in turn leads to a tremendous decrease in the expression of NF-kappaB target gene products, such as c-myc, cyclin D1, Bcl-2, Bcl-xL (Wang et al., 2010). DHA was also shown to exhibit significant anti-cancer activity against the renal epithelial LLC cell line. In addition, DHA was shown to induce apoptosis of LLC cells and influenced the expression of the vascular endothelial growth factor (VEGF) receptor KDR/flk-1. Furthermore, in both tumor xenografts, a greater degree of growth inhibition was achieved when DHA and chemotherapeutic drugs were used in combination. The combined effect of DHA administered with chemotherapy drugs on LLC tumor metastasis was shown to be significant (Zhou et al., 2010).
The effect of DHA was investigated using in vitro/in vivo optical imaging combined with cell/tumor growth assays of the pancreatic cancer cell line BxPc3-RFP which stably expresses red fluorescence protein. DHA inhibited the proliferation and viability of pancreatic cancer cells in a dose-dependent manner and induced apoptosis. The results of this experiment demonstrated DHA-induced down-regulation of PCNA and Bcl-2, and up-regulation of Bax. VEGF expression was down-regulated by DHA in cells under normoxic, but not hypoxic, conditions. The anti-angiogenic effect of DHA appears to be a complicated process (Aung et al., 2011). DHA was shown to significantly inhibit NF-κB DNA-binding activity, which in turn results in a tremendous decrease in the expression of NF-κB-targeted pro-angiogenic gene products such as VEGF, IL-8, COX-2, and MMP-9 in vitro: These findings suggest that DHA could be developed as a novel agent against pancreatic cancer (Wang et al., 2011). Additional supporting evidence of the potential of DHA to be used as an anti-pancreatic cancer agent were shown through a DHA driven up-regulation of glucose-regulated protein 78 (GRP78), which is known to be involved in endoplasmic reticulum stress (ER stress),. Further study demonstrated that DHA could enhance expression of GRP78 as well as the growth arrest and DNA-damage-inducible gene 153 at both the mRNA and protein levels. These studies suggest that redox imbalance may result in DHA-induced ER stress, which may contribute, at least in part, to its anti-cancer activity (Lu et al., 2011).
Artesunate (AS)
AS has been shown to inhibit the growth of Kaposi’s sarcoma cells, a highly angiogenic multifocal tumor, and the degree of cell growth inhibition correlated with the induction of apoptosis. AS was also shown to inhibit the growth of normal human umbilical endothelial cells and of KS-IMM cells that were established from a Kaposi’s sarcoma lesion obtained from a renal transplant patient. The inhibition of cell growth correlated with the induction of apoptosis in KS-IMM cells. Apoptosis was not observed in normal endothelial cells, which showed drastically increased cell doubling times upon AS treatment (Dell’Eva et al., 2004).
AS has been shown to greatly inhibit cell proliferation and differentiation of endothelial cells in a dose-dependent manner in the range of 12.5-100 µM. AS was also shown to reduce Flt-1 and KDR/flk-1 expression of endothelial cells when dosed in vitro in a range of 0.1-0.5 µM. In subsequent studies by the same author, the AS-driven apoptosis of a human microvascular dermal endothelial cell line was studied. The apoptosis was detected utilizing a morphological dual staining assay composed of ethidium bromide and acridine orange as well as a DNA fragmentation TUNEL assay quantified by a flow cytometric propidium iodide (PI) assay. The results suggest that the anti-angiogenic effect induced by AS treatment might occur by the induction of cellular apoptosis (Huan-huan et al., 2004). In addition, the inhibitory effect of AS on in vitro angiogenesis was tested using aortic cells cultured in a fibrin gel. AS was shown to effectively suppress the stimulating angiogenic ability of chronic myeloid leukemia cells (line K562) when the K562 cells were pretreated for 48 h with AS in a time-dependent manner (days 3-14). AS treatment was also found to decrease the VEGF level in chronic myeloma K562 cells, even at a lower concentration (2 µmol/l, P < 0.01). (Zhou et al., 2007).
The addition of Fe(II)-glycine sulfate and transferrin has been shown to enhance the cytotoxicity (10.3-fold) of free AS in vitro. AS microencapsulated in maltosyl-ß- cyclodextrin, and ARTs were tested against CCRF-CEM leukemia and U373 astrocytoma cells in vitro (Efferth et al., 2004). Treatment with AS at more than 2.5 µM for 48 h inhibited the proliferation of human vein endothelial cells (HUVEC) in a concentration dependent manner using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) based growth proliferation assay (p < 0.05). The IC50 value of this growth inhibition assay was 20.7 µM, and HUVEC cells were also shown to be growth inhibited by 88.7% after treatment with 80 µM AS (Chen et al., 2004b).
AS at low concentration was shown to significantly decrease VEGF and Ang-1 secretion by human multiple myeloma cells (line RPMI8226, P < 0.05), which correlated well with the reduction of angiogenesis induced by the myeloma RPMI8226 cells. This study also showed that AS down-regulated the expression of VEGF and Ang-1 in RPMI8226 cells and reduced the activation of extracellular signal regulated kinase 1 (ERK1) as well. Therefore, AS has been shown to block ERK1/2 activation, downregulate VEGF and Ang-1 expression and inhibit angiogenesis induced by human multiple myeloma RPMI8226 cells. Combined with previous published data, the results from this study supports the hypothesis that AS possesses potential anti-myeloma activity (Chen et al., 2010a).
AS has also been shown to decrease the secretion of VEGF and IL-8 from TNFα- or hypoxia-stimulated rheumatoid arthritis fibroblast-like synoviocyte (line RA FLS) in a dose-dependent manner. In addition, AS treatment resulted in the inhibition of TNFα- or hypoxia-induced nuclear expression and translocation of HIF-1α. AS treatment was shown to prevent Akt phosphorylation, but there was no evidence that phosphorylation of p38 and ERK was averted. TNFα- or hypoxia-induced secretion of VEGF and IL-8 and expression of HIF-1α were hampered by treatment with the PI3 kinase inhibitor LY294002, suggesting that inhibition of PI3 kinase/Akt activation might inhibit VEGF, IL-8 secretion, and HIF-1α expression induced by TNFα or hypoxia. Therefore, AS has been shown to inhibit angiogenic factor expression in the RA FLS cell line, and this latest study provides new evidence that, as a low-cost agent, AS may have therapeutic potential for rheumatoid arthritis (He et al., 2011).
Using a polyploid cell line, research on the role of AS in impacting cell cycle arrest was assessed. The results of this study show that AS treatment of polyploid cells resulted in a dose-dependent decreases in cell number, which was associated with either increased cytotoxicity or cytostasis. Of the two possibilities, cytostasis, a simultaneous arrest at all phases of the cell cycle, appeared to be a more likely possibility. This deduction was supported by molecular profiling, which showed reductions in cell cycle transit proteins. AS appeared to maintain cells in this arrested state, however, reculturing these treated cells in drug-free medium resulted in significant reductions in cell viability. Taken together, these observations indicate AS and its related compounds may be effective for the treatment of polyploid tumors, and that activity is related to the cell cycle schedule. Therefore, it is important to carefully select the most appropriate schedule to maximize AS efficacy when using AS as a primary or adjuvant anti-tumor therapy (Liu et al., 2011)
3.1.2. In vivo anti-angiogenic effects of ART and its derivatives
There are many reports discussing the in vivo anti-cancer activity of ARTs which may provide insight into the potential activity of ARTs as anti-cancer agents.
Artemisinin (ART)
The effect of ART on tumor growth, lymphangiogenesis, metastasis and survival in mouse Lewis lung carcinoma (LLC) models was examined. The results of this study showed that orally administered artemisinin inhibited lymph node and lung metastasis and prolonged survival without retarding tumor growth. ART-treated mice showed significant decreases in lymph node metastasis, tumor lymphangiogenesis and expression of VEGF-C as compared to control mice. (Wang et al., 2008).
Dihydroartemisinin (DHA)
The anti-angiogenic activity of DHA in vitro and in vivo, and investigated DHA-induced apoptosis in human umbilical vein endothelial cells (HUVEC). DHA markedly reduced VEGF binding to its receptors on the surface of HUVEC. The expression levels of two major VEGF receptors, Flt-1 and KDR/flk-1, on HUVEC were lower following DHA treatment as shown by an immunocytochemical staining assay. The in vivo anti-angiogenic activity was evaluated in the chicken chorioallantoic membrane (CAM) neovascularization model. DHA significantly inhibited CAM angiogenesis at low concentrations (5-30 nmol/100 microl per egg). This group also investigated both qualitatively and quantitatively the induction of HUVEC apoptosis by DHA. A dose-related (5-80 µM) and time-dependent (6-36 h) increase in DHA-induced HUVEC apoptosis was observed by flow cytometry. These results suggest that the anti-angiogenic effect induced by DHA might occur by induction of cellular apoptosis and inhibition of expression of VEGF receptors. These findings and the known low toxicity of DHA indicate that it might be a promising candidate angiogenesis inhibitor (Chen et al., 2004a).
The anti-angiogenic effect of DHA on pancreatic cancer was assessed using BxPC-3 xenografts subcutaneously established in BALB/c nude mice. DHA demonstrated remarkable activity against pancreatic cancer studies concuted in vivo. DHA treatment resulted in reduced tumor volume and decreased microvessel density, and there were additional transcriptional effects demonstrated in these studies as well regarding the expression of NF-κB-related pro-angiogenic gene products which were down-regulated. This finding of relating to the inhibition of NF-κB activation is likely one of the mechanisms involved in DHA anti-angiogenic activity against human pancreatic cancer. This suggests that DHA could be developed as a novel agent against pancreatic cancer (Wang et al., 2011). In a further study, the co-administration of the chemotherapeutic agent gemcitabine with DHA was shown to result in remarkably enhanced anti-tumor effects, as demonstrated by significantly increased apoptosis, as well as a decreased Ki-67 index, reduced NF-kappaB activity, reduced downstream angiogenic gene products, and predictably, significantly reduced tumor volume. The authors conclude that inhibition of gemcitabine-induced NF-kappaB activation is one of the mechanisms by which DHA could promote its anti-tumor effect on pancreatic cancer (Wang et al., 2010).
Artesunate (AS)
The anti-angiogenic effect in vivo of artesunate was evaluated in nude mice f implanted with human ovarian cancer cells (HO-8910). The effects of artesunate on angiogenesis in this in vivo study were evaluated by immune-histochemical staining for microvessel associated antigens (CD31), VEGF and the VEGF receptor KDR/flk-1. AS significantly inhibited angiogenesis in a concentration-dependent form in the range of 0.5-50 µM. The IC50 of AS for HUVE cells was 21 µM. Growth of the xenograft tumor was decreased and microvessel density was reduced following drug-treatment with no apparent toxic effects on the nude mice. AS administration was shown to dramatically reduce VEGF expression on tumor cells and KDR/flk-1 expression on endothelial cells as well as tumor cells. Accordingly, these results support the hypothesis that AS is capable of inhibiting angiogenesis in vitro and in vivo. These findings together with the known low toxicity of AS are clues that AS may be a promising angiogenesis inhibitor (Chen et al., 2004c).
Further studies on the anti-angiogenic effects of AS have been conducted in vivo and in vitro. The anti-angiogenic effect of AS in vivo was evaluated utilizing the chicken chorioallantoic membrane (CAM) neovascularization model. At low concentrations of 10 nM/100 µl/egg, AS was shown to significantly inhibit CAM angiogenesis, and completely inhibited angiogenesis at concentrations of 80 nM/100 µl/egg. The results of this study suggest that the anti-angiogenic effect induced by AS might occur by the induction of cellular apoptosis. These findings and the known low toxicity of AS support the hypothesis that AS might be a promising candidate as an angiogenesis inhibitor (Huan-huan et al., 2004). Similarly, AS was shown to significantly impair primary tumor growth and metastasis in the chicken embryo metastasis (CAM) model where AS was shown to suppress invasion and metastasis of non-small cell lung cancer (NSCLC) cells. The transcriptional findings of these experiments showed AS treatment reduced transcription of u-PA, MMP-2 and MMP-7, supporting the hypothesis that AS has promise as a novel therapeutic for NSCLC (Rasheed et al., 2010).
Also, AS has been studied in a variety of tumor models as a potential antitumor drug. In one study of vascularization, a critical element of tumor metastasis, AS was shown to strongly reduce angiogenesis of Kaposi’s sarcoma cells in vivo by inhibiting vascularization in Matrigel plugs injected subcutaneously into syngenic mice. This data suggests that AS represents a promising candidate drug for the treatment of the highly angiogenic Kaposi’s sarcoma. As a low-cost drug, it might be of particular interest for use in areas of the world where Kaposi’s sarcoma is highly prevalent. (Dell’Eva et al., 2004).
The efficacy of AS, as an anti-cancer agent, to reduce tumor growth was studied in rats given AS subcutaneously at a dose of 50 mg/kg/day and at a dose of 100 mg/kg/day for 15 days. The results of this experiment showed animals with AS treated tumors showed a reduction in tumor growth by 41%, in the 50 mg/kg treatment group and 62% in the 100 mg/kg treatment group. The density of micro-vessels which was used as a measure of angiogenic activity in the tumors of animals treated with 100 mg/kg of AS daily was at least four times lower than in the control group (Chen et al., 2004b). The anti-angiogenic activity of AS in vivo was also evaluated in nude mice implanted with a human ovarian cancer cell line (HO-8910). Evaluation of angiogenesis in the AS treated and control animals with an ovarian cancer xenograft were determined through immunohistochemical staining for microvessel formation (CD31), VEGF and the VEGF receptor KDR/flk-1. Tumor growth was noted to be decreased, and the density of the tumor microvessels was reduced following AS treatment with no apparent toxicity to the animals (Chen et al., 2004a, 2004b).
The anti-angiogenic effect of AS was further evaluated in vivo in the chicken chorioallantoic membrane (CAM) neovascularization model. The results showed that stimulating angiogenic activity was decreased in response to the treatment of myeloblastic K562 cells with ART, and tumor growth was inhibited when K562 cells were pretreated with ART in a dose-dependent manner (3-12 µmol/l). Further analyses of the level of VEGF expression by Western blot and also assays of VEGF mRNA by RT-PCR in K562 cells showed that ART could inhibit VEGF expression, and the inhibition correlated well with the level of VEGF secreted in the culture medium. These findings suggest that AS may have potential as a treatment for chronic myelogenous leukemia (CML) or as an adjunct to standard chemotherapeutic regimens (Zhou et al., 2007).
3.1.3. Anti-angiogenic effects of novel ARTs and ART-like compounds
Artesunate has been shown to exhibit anti-angiogenic, anti-tumorigenic and anti-viral properties in addition to its known antimalarial properties. The array of activities of the ARTs, and the recent emergence of malaria resistance to AS, prompted one group to synthesize and evaluate several novel ART-like derivatives. Sixteen distinct derivatives were therefore synthesized, and the in vitro cytotoxic effects of each were tested with different cell lines. The in vivo anti-angiogenic properties were evaluated using a zebrafish embryo model. This groupreported the identification of several novel ART-like compounds that are easily synthesized, stable at room temperature, may overcome drug-resistance pathways and are more active in vitro and in vivo than the commonly used AS. These promising findings raise the hopes of identifying safer and more effective strategies to treat a range of infections and cancer (Soomro et al., 2011).
Twelve ART acetal dimers were synthesized and tested for antitumor activity against 60 in vitro tumor cell lines compiled by the National Cancer Institute (NCI), producing a mean GI50 concentration between 8.7 (least active) and 0.019 µM (most active). The significant activity of the compounds in this preliminary screen led to additional in vitro antitumor and anti-angiogenesis studies. Several active dimers were also evaluated in the in vivo NCI hollow fiber assay followed by a preliminary xenograft study. The title compounds were found to be active against solid tumor-derived cell lines and showed good correlation with other artemisinin-based molecules in the NCI database (Galal et al., 2009).
In addition, various thioacetal ART derivatives can inhibit the angiogenesis and might be angiogenesis inhibitors. In particular, 10 alpha-phenylthiodihydroartemisinins, 10 beta-benzenesulfonyl-9-epi-dihydroartemisinin and 10 alpha-mercaptodihydroartemisinin exhibit strong growth inhibition activity against HUVEC proliferation. Compound 11 have a good inhibitory activity upon HUVEC tube formation, and 5 and 11 show a strong inhibitory effect on angiogenesis using CAM assay at 5 µg/egg by 90% (Oh et al., 2004).
Artemisone is a novel 10-alkylamino derivative which is not metabolized to DHA. It was selected as a clinical drug candidate on the basis of its potency in vitro against Plasmodium falciparum and its lack of detectable neurotoxicity in both in vitro and in vivo screens. Artemisone was tested in vitro and in vivo for anti-angiogenic effects which may support its use as an anti-angiogenic agent as an adjunct to standard tumor chemotherapy. The various studies of artemisone’s anti-angiogenic activity include proliferation of human endothelial cells and their migration on a fibronectin matrix, the sprouting of new vessels from rat aorta sections grown in collagen, and the production of pro-angiogenic cytokines such as vascular endothelial growth factor (VEGF) and interleukin-8 (CXCL-8). The data showed that artemisone is significantly less anti-angiogenic than DHA in all the experimental models tested, suggesting that artemisone will be safer to use than the current clinical artemisinins during pregnancy for an antimalarial indication but perhaps less efficacious for an anti-angiogenic indication as part of a anti-cancer regimen (D’Alessandro et al., 2007).
3.2. Mechanistic perspectives for the anti-angiogenic activities of ARTs
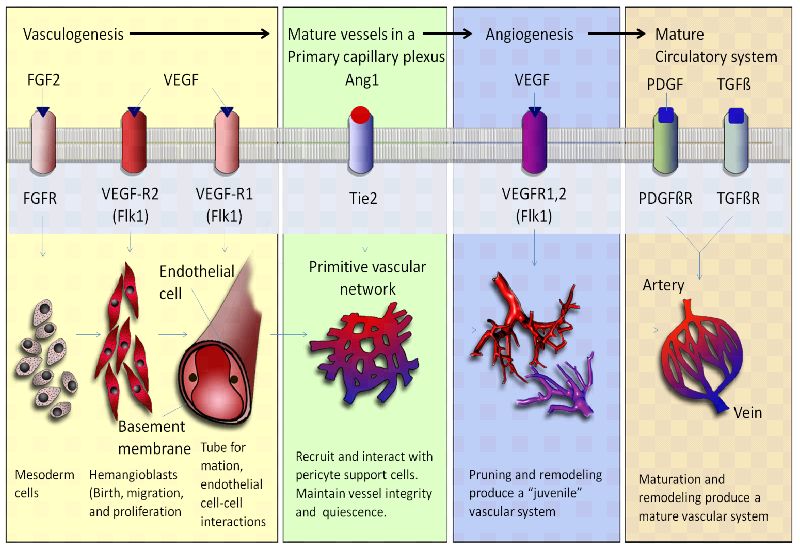
Angiogenesis and vasculogenesis refer to the growth of blood vessels. Angiogenesis is the growth most often associated with repair of damaged vessels or the growth of smaller blood vessels, while vasculogenesis is the process by which the primary blood system is being created or changed. Vasculogenesis occurs during the very early developmental stages of an organism when the blood vessel pathways are created. Angiogenesis, while a similar process, does not depend on the same set of genes as vasculogenesis, and this process is activated instead in the presence of an injury to a blood vessel. In the last three decades, considerable research has been reported that supports the hypothesis that tumor growth and metastasis require angiogenesis. Angiogenesis, the proliferation and migration of endothelial cells resulting in the formation of new blood vessels, is an important process for the progression of tumors (Figure 3). ARTs have been shown in a number of published reports to have anti-angiogenic effects.
As malignant tissues grow, metastases and solid tumors require extra blood supply for thriving and survival. Thus, cancer cells induce neovascularization by regulating proteins and pathways involved in the generation and restructure of new vasculature. Angiogenesis process leads to enhanced proliferation of endothelial cells through induction of VEGF, fibroblast growth factor (FGF), its receptors, and cytokines. This event occurs via multiple effects including hypoxia-driven activation of expression of HIF-1α and the aryl hydrocarbon receptor nuclear translocator (ARNT). Angiogenesis control is mediated by angiostatin, endostatin, thrombospondin, TIMPs, PAI-1, and others. Due to their role in tumor survival, the pro-angiogenic factors and the molecules involved in their regulatory networks are relevant drug targets (Crespo-Ortiz and Wei, 2012)
Cancers are capable of spreading through the body by two mechanisms: invasion and metastasis. Invasion is the direct migration and penetration by cancer cells into neighboring tissues. Metastasis is the ability of cancer cells to penetrate into lymphatic and blood vessels, circulate through the bloodstream, and then grow in a new focus (metastasize) in normal tissues elsewhere in the body. Without a connection to a network of blood vessels, a tumor can only grow to about the size of a pinhead (1-2 mm), that is to say a tumor is in a vascular, quiescent status. When a subgroup of cells within the tumor switches to an angiogenic phenotype by changing the local equilibrium between positive and negative regulators of angiogenesis, tumor starts to grow rapidly and becomes clinically detectable. Anti-angiogenesis therapy is a novel approach in cancer treatment and prevention of tumor metastasis. It is therefore expected that angiogenesis inhibitors may be clinically useful for the treatment of tumors.
3.2.1. Anti-cancer mechanism of ARTs on angiogenesis-related genes
Angiogenesis involves tissue restructuring, and genes that regulate angiogenesis, such as chemokine receptors, can also affect tumor metastasis. A vital requirement of neovasculogenesis is endothelial mitosis, which occurs in response to activation by pro-angiogenic signaling from VEGF and its receptors. Three human genes encode for VEGF (VEGFA, VEGFB and VEGFC) and splice variants add more heterogeneity to the biological actions of the VEGF gene family (Tischer et al., 1991). Analysis of VEGF transcripts in cultured vascular smooth muscle cells by PCR and cDNA cloning revealed three different forms of the VEGF coding region, which has also been previously reported in HL60 cells. The three forms of the human VEGF protein chain predicted from these coding regions are 189, 165, and 121 amino acids in length. Comparison of cDNA nucleotide sequences with sequences derived from human VEGF genomic clones indicates that the VEGF gene is split among eight exons and that the various VEGF coding region forms arise from this gene by alternative splicing. Analysis of the VEGF gene promoter region revealed a single major transcription start, which lies near a cluster of potential Sp1 factor binding sites. Northern blot analysis demonstrated that the level of VEGF transcripts is elevated in cultured vascular smooth muscle cells after treatment with the phorbol ester 12-O-tetradecanoyl-phorbol-13-acetate (Tischer et al., 1991).
In a study using an US National Cancer Institute (NCI) panel of 60 tumor cell lines, ART and related compounds displayed anti-angiogenic activities based on the altered expression of genes implicated in angiogenesis. The mRNA expression data of angiogenesis-related genes correlated well with the 50% growth inhibition concentration values for eight ARTs (ART, AS, arteether, artemisetene, arteanuine B, dihydroartemisinylester stereoisomers 1 and 2). The constitutive expression of 30 different genes correlated significantly with the cellular response to ARTs. The finding that drug sensitivity and resistance of tumor cells could be predicted by the mRNA expression of angiogenesis related genes supports the hypothesis that the antitumor activity of ARTs may be due, at least in part, by inhibition of tumor angiogenesis. As many chemo-preventive drugs exert anti-angiogenic features, ARTs might also have a chemo-preventive effect in addition to their cytotoxic effects (Anfosso et al., 2006).
These findings are consistent with previous published work (Wartenberg et al., 2003) showing an artemisinin-dependent decrease in expression levels of hypoxia-inducible factor 1a (HIF-1a; H1F1A), which is known to be a transcriptional activator of VEGFA and is critical in neovasculogenesis in hypoxic tissues. The inhibition of angiogenesis by ART (at a concentration of 12 mM) involving VEGF and HIF-1a was also demonstrated in leukemic and glioma cells (Huang et al., 2008; Zhou et al., 2007). Loss of HIF-1α and VEGF expression by artemisinin appears to depend on ROS as co-treatment with free-radical scavengers such as vitamin E and mannitol reversed the effects of artemisinin (Wartenberg et al., 2003). The sensitivity and resistance of these tumor cells has been shown to correlate with mRNA expression of angiogenesis-related genes. This suggests that the anti-tumor effects of ARTs are potentially due to their role in inhibiting tumor angiogenesis (Anfosso et al., 2006). The finding that tumor cell drug sensitivity and resistance could be predicted by mRNA expression of angiogenesis-related genes supports the hypothesis that artemisinins their anti-tumor effects at least in part by inhibition of tumor angiogenesis.
In addition, an investigation to determine the sensitivity and resistance of cancer cells towards AS was conducted. The gene-hunting approach applied by us delivered several novel candidate genes that may regulate the response of cancer cells to AS. These results merit further investigations to prove the contribution of these genes for AS resistance. Study demonstrated that AS was no inhibitor of ABC transporters ABCB1 and ABCG2. Although AS may exhibit specific inhibitory functions towards particular ABC transporters, but not towards a wide spectrum of several different ABC transporters. This approach showed that response of tumor cells towards AS is multi-factorial in nature and is determined by gene expression associated with AS sensitivity on the one hand and with gene expression associated with AS resistance on the other hand (Sertel et al., 2010).
3.2.2. Anti-proliferative mechanisms of ARTs
The anti-cancer mechanism of ARTs is likely to be related to the cleavage of the iron- or heme-mediated peroxide bridge, followed by the generation of ROS (Mercer et al., 2011; Zhang et al., 2010). The anti-cancer potential of ARTs is possibly connected to the expression of TfR. The synergism of AS and iron (II)-glycine sulfate co-treatment is unsuitable for all types of tumor cells. Endoplasmic reticulum stress is partially involved in some cases of ARTs-mediated anti-proliferation (Lu et al., 2010; Stockwin et al., 2009).
In normal cells, cyclin-dependent kinases (CDK) are the proteins translating signals in order to guide cells through the cell-division cycle. Normal growth relies on the ability to translate signals in order to replicate and divide in an effective manner (McDonald and El-Deiry, 2000). Uncontrolled proliferation in cancer cells is known to result from mutations inducing amplification of growth signals, deregulation of checkpoints, and loss of sensitivity to growth inhibitors. Abnormal cell growth is also triggered by deregulation of programmed cell death or apoptosis (Vogelstein and Kinzler, 2004). ARTs have been shown to effectively induce cell growth arrest in cancer lines either by disrupting the cell cycle kinetics or by interfering with proliferation-interacting pathways.
DHA and AS are very potent growth inhibitors, and multiple studies have demonstrated that DHA is the most potent anti-cancer artemisinin-like compound (DHA > AS > AM) (Efferth et al., 2003; Woerdenbag et al., 1993). Recently, artemisone has shown impressive antitumor efficacy in 7 cells lines including melanoma and breast cancer cells (Gravett et al., 2010). ART compounds have been shown to exert cytostatic and cytotoxic action on cancer cells (Efferth et al., 2003; Hou et al., 2008). ART-induced growth arrest has been reported at all cell cycle phases; however, arrest at the G0/G1 to S transition seems to be more commonly affected (Efferth et al., 2003). Arrest at all cell cycle phases at the same time has been interpreted as a cytostatic effect. Disruption of the cell cycle at G2/M was observed after DHA treatment in osteosarcoma, pancreas, leukemia (Yao et al., 2008) and ovarian cancer cells (Jiao et al., 2007). Similarly, AS interferes with G2 in osteosarcoma, ovarian, and other different cancer lines (Ji et al., 2011).
Several ART derivatives displayed higher cytotoxicity to murine bone marrow cells than to murine Ehrlich ascites tumor cells in a clonogenic assay (Beekman et al., 1998). The IC50 values for HeLa cervical cancer cells, uterine chorion cancer JAR cells, embryo transversal cancer RD and ovarian cancer HO-8910 cell lines after 48-h treatment with ART and DHA ranged from 15 to 50 µM and from 8 to 33 µM, respectively (Chen et al., 2003). ART potentiated the differentiation of 1α, 25-dihydroxyvitamin-D3-induced HL-60 leukemia cell predominantly into monocytes and all-trans RA-induced cell differentiation into granulocytes, respectively (Kim et al., 2003). Signal transducers involved in the differentiation process, such as extracellular-signal regulated kinase (ERK) and protein kinase C ß1 (PKCB1) were affected by ART.
Inhibition of proliferation may also be attributed to down-regulation of interacting proteins targeting multiple pathways (Firestone and Sundar, 2009). It has been shown that DHA treatment of pancreatic cells (BxPC3, AsPC-1) inhibited cell viability by decreasing the levels of proliferating cell nuclear antigen (PCNA) and cyclin D with parallel increase in p21 (Chen et al., 2009). Another study in the same system showed that DHA counters NF-κB factor activation leading to inhibition of its targets in the proliferation (c-myc, cyclin D) and apoptotic pathways (Bcl2, Bcl-xl) (Wang et al., 2010). In prostate cancer, DHA has been shown to induce cell cycle arrest by disrupting the interaction of Sp1 (specificity protein 1) and the CDK4 promoter (Willoughby et al., 2009). Dissociation of the Sp1-CDK4 complex promotes caspase activation and cell death. In addition, another study has identified AS as a topoisomerase II inhibitor which inhibits growth by interaction with multiple pathways (Youns et al., 2009).Overall, a wide body of research supports the hypothesis that ARTs are capable of interfering with several pathways known to be involved in neoplasia.
3.2.3. Anti-VEGF mechanisms of ARTs
Angiogenesis is promoted by numerous factors including cytokines such as VEGF, bFGF, PDGF and others. It is negatively regulated by angiostatin, endostatin, thrombospondin, TIMPs and other factors. The factors that are produced in tumor cells as well as in surrounding stromal cells act in a balance to promote either pro-angiogenic or anti-angiogenic processes. Among the cytokines for regulating angiogenesis, VEGF and angiopoietin-1 (Ang-1) have specific modulating effects on the growth of vascular endothelial cells, and they play a key role in the process of angiogenesis (Thurston 2002). VEGF is a homodimeric 34-42 kDa, a heparin-binding glycoprotein with potent angiogenic, mitogenic and vascular permeability-enhancing activities specific for endothelial cells. Two receptor tyrosine kinases have been described as putative VEGF receptors, Flt-1 and KDR. Flt-1 (fms-like tyrosine kinase), and KDR (kinase-insert-domain-containing receptor) proteins have been shown to bind VEGF with high affinity.
ART and DHA have been shown to significantly inhibit angiogenesis in a dose-dependent manner as demonstrated by measurement of the proliferation, migration and tube formation of human umbilical vein endothelial (HUVE) cells (Chen et al., 2003). DHA was shown to markedly reduce VEGF binding to its receptors on the surface of HUVE cells and reduced the expression levels of two major VEGF receptors, Flt-1 and KDR/flk-1, on HUVE cells. ART derivatives also inhibited HUVE cell tube formation and exhibited anti-angiogenic effects (Oh et al., 2004). By utilizing the chicken chorioallantoic membrane (CAM) culture technique, it is possible to detect the microangium-like structures formed by in vitro cultivated arterial rings associated with angiogenesis. By using this method, AS has been shown to also have anti-angiogenic effects. Treatment with AS significantly inhibited chicken chorioallantoic membrane (CAM) angiogenesis, proliferation, and differentiation of human microvascular dermal endothelial cells in a dose-dependent manner and reduced Flt-1 and KDR/flk-1 expression (Huan-Huan et al., 2004).
Tumor hypoxia activates the transcription factor hypoxia inducible factor-1α (HIF-1α). This adaptation increases tumor angiogenesis to support the survival of poorly nourished cancer cells. Hypoxic tumors are resistant to radiation and many anti-cancer agents. HIF-1α is activated during angiostatic therapy, and HIF-1α has also been shown to up-regulate the expression of transferrin receptors. Since ART is selectively toxic to iron-loaded cells, radio and drug-resistant tumors might be selectively susceptible to attack by a treatment strategy consisting of iron-loading and ART treatment (Li et al., 2008; Zhou et al., 2007).
These findings are consistent with previous findings (Wartenberg et al., 2003) that noted ART-dependent decreases in expression levels of HIF-1α. HIF-1α is known to be a transcriptional activator of VEGF, and it plays a crucial role in neo-vasculogenesis in hypoxic tissues. ART treatment of leukemic and glioma cells in vitro at a concentration of 12 mM was shown in another study to inhibit angiogenesis. This ART driven angiogenesis inhibition was shown to involve suppression of VEGF and HIF-1α expression at the transcriptional level. (Huang et al., 2008; Zhou et al., 2007). Loss of HIF-1α and VEGF expression after ART treatment appears to be dependent on production of ROS because co-treatment with free-radical scavengers such as vitamin E and mannitol reversed the effects of ART (Wartenberg et al., 2003).
In vitro, VEGF is a potent endothelial cell mitogen. In cultured endothelial cells, VEGF has been shown to activate phospholipase C and induce rapid increases of free cytosolic Ca2+. VEGF has also been shown to stimulate the release of von Willebrand factor from endothelial cells and induce expression of tissue factor activity in endothelial cells as well as in monocytes, and. VEGF has been shown to be involved in the chemotaxis of monocytes and osteoblasts. In vivo, VEGF can induce angiogenesis as well as increase microvascular permeability. As a vascular permeability factor, VEGF acts directly on the endothelium and does not degranulate mast cells. It promotes extravasation of plasma fibrinogen, leading to fibrin deposition which alters the tumor extracellular matrix. The modified extracellular matrix subsequently promotes the migration of macrophages, fibroblasts and endothelial cells. Based on its in vitro and in vivo properties, VEGF is believed to play important roles in inflammation and also in normal and pathological aspects of angiogenesis, a process that is associated with wound healing, embryonic development, growth, and metastasis of solid tumors. Elevated levels of VEGF have been reported in synovial fluids of rheumatoid arthritis patients and in sera from cancer patients.
In the last three decades, there is a growing body of evidence on the role of angiogenesis in tumor growth and metastases of tumors (Firestone and Sundar, 2009). Angiogenesis can be divided into a series of temporally regulated responses, including induction of proteases, migration of endothelial cells, cell proliferation and differentiation. This is a highly complex process, in which a number of cytokines and growth factors released by endothelial cells, tumor cells and matrix cells are involved. The expression of VEGF has been suggested to be related to some fundamental features of solid tumors, such as the growth rate, the density of tumor microvessels, and the development of tumor metastases.
It is interesting to note that torilin, another sesquiterpene (derived from the fruits of Torilis japonica), has also been shown to be a potent anti-angiogenic factor which also inhibits blood vessel formation by disrupting VEGFA expression. A similar finding was also shown by using DHA (Kim et al., 2007). Hence, the ability of ART to inhibit angiogenesis may be due to its chemical nature as a sesquiterpene. Another compelling finding is that other phytosesquiterpene lactones, such as costunolide from Saussurea lappa, can inhibit KDR signaling (Jeong et al., 2002). Comparisons with other sesquiterpenes may shed more light on the unique features of the anti-cancer actions of ART, and potentially lead to better angiostatic drug design. Taken together, ART and its derivatives, and other sesquiterpene lactones, have been shown to have potent anti-angiogenic effects in tumor cells. These observations have many implications in terms of cancer therapy as well as cancer prevention since angiogenesis is a promotional event.
3.2.4. Other anti-angiogenic mechanisms of ARTs
Anti-cancer activity of ARTs has been reported both in vitro and in vivo. The inhibitory effects of ART on the migratory ability of melanoma cell lines (A375P and A375M) were analyzed, and the results demonstrated that ART induces cell growth arrest in the A375M cell line, and affects the viability of A375P melanoma cells through both cytotoxic and growth inhibitory effects. In addition, ART was shown to affect the migratory ability of A375M melanoma cells by reducing metalloproteinase 2 (MMP-2) production and down-regulating alpha v beta 3 integrin expression (Buommino et al., 2009). Other studies, however, showed ART was not effective in inhibiting proliferation of other tumor cell lines such as MCF7, a breast adenocarcinoma cell line, and MKN, a gastric carcinoma line.
Similarly, ARTs have been shown to inhibit Matrigel invasion of 6 non-small cell cancer (NSCLC) cell lines and inhibited urokinase-type plasminogen activator (u-PA) activity, -protein and -mRNA expression. Furthermore, in a PCR-metastasis array, ARTs were shown to inhibit the expression of several matrix metalloproteinases (MMPs), especially MMP-2 and MMP-7 mRNA/protein. In luciferase reporter assays, ARTs were shown to down-regulate MMP-2-, MMP-7- and u-PA-promoter/-enhancer activity, in parallel to AP-1- and NF-kB-transactivation. Si-RNA knockdown of u-PA, MMP-2 and MMP-7 abolished ART’s ability to inhibit invasion, further supporting hypotheses of the anti-cancer activity of ARTs In conclusion, this study showed that ART treatment suppresses invasion and metastasis in NSCLC, specifically targeting transcription of u-PA, MMP-2 and MMP-7. These studies all support the utility of ART compounds as novel therapeutic agents or adjunct therapies for NSCLC (Rasheed et al., 2010).
DHA displayed significant anti-proliferative activity in human colorectal carcinoma HCT116 cells, which may be attributed to its induction of G1 phase arrest and apoptosis. To further elucidate the mechanism of action of DHA, a proteomic study employed two-dimensional gel electrophoresis (2-DE) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed. Glucose-regulated protein 78 (GRP78), which is related with endoplasmic reticulum stress (ER stress), was identified to be significantly up-regulated after DHA treatment. Further study demonstrated that DHA enhanced expression of GRP78 as well as growth arrest and DNA-damage-inducible gene 153 (GADD153, another ER stress-associated molecule) at both mRNA and protein levels. DHA treatment also led to accumulation of GADD153 in cell nucleus. Moreover, pretreatment of HCT116 cells with the iron chelator deferoxamine mesylate salt (DFO) abrogated induction of GRP78 and GADD153 upon DHA treatment, indicating iron is required for DHA-induced ER stress. This result is consistent with the fact that the anti-proliferative activity of DHA is also mediated by iron. Accordingly, it is possible that a redox imbalance may be the mechanism behind DHA-induced ER stress, which may contribute, at least in part, to its anti-cancer activity (Lu et al., 2011).
DHA has been shown to enhance gemcitabine-induced growth inhibition and apoptosis in both BxPC-3 and PANC-1 cell lines. The mechanism is at least partially due to DHA’s role in deactivating gemcitabine-induced NF-kappaB activation, which, in turn, so as to dramatically decreases the expression of its target gene products, such as c-myc, cyclin D1, Bcl-2, Bcl-xL. In vivo studies have shown that, gemcitabine also manifested remarkably enhanced anti-tumor effects when combined with DHA, as demonstrated by significantly increased apoptosis, as well as decreased Ki-67 index, NF-kappaB activity and its related gene products, and predictably, significantly reduced tumor volume. The inhibition of gemcitabine-induced NF-kappaB activation is one of the mechanisms that is known by which DHA dramatically promotes its anti-tumor effect on pancreatic cancer (Wang et al., 2010).
Embryotoxicity appears to be connected with defective angiogenesis and vasculogenesis in certain stages of embryo development. This may prevent the use of ART derivatives in malaria during pregnancy, when both mother and fetus are at high risk of death. Artemisone is a novel 10-alkylamino derivative which is not metabolized to DHA. It was selected as a clinical drug candidate on the basis of its high efficacy against Plasmodium falciparum in vitro and its lack of detectable neurotoxicity in both in vitro and in vivo screens. A comparative study of the anti-angiogenic properties of both artemisone and dihydroartemisinin in different model systems was conducted. In this study, the effects of both artemisone and DHA were evaluated by measuring the proliferation of human endothelial cells and their migration on a fibronectin matrix, the sprouting of new vessels from rat aorta sections grown in collagen, and the production of pro-angiogenic cytokines such as VEGF and interleukin-8 (CXCL-8). The data show that artemisone is significantly less anti-angiogenic than DHA in all the experimental models, suggesting that it will be safer to use than the current clinical ARTs during pregnancy (D’Alessandro et al., 2007).
3.3. Anti-cancer clinical trials and case treatments of ARTs
Antitumor activity of ARTs has also been documented in human trials and individual clinical cases. ART, AM and AS have been used in cancer therapy, and they have been shown to be well tolerated without significant side effects (Table 3).
3.3.1. Clinical trials of ARTs as anti-cancer agents
Clinical evidence has accumulated showing that ART-derived drugs have promise for treatment of laryngeal carcinomas, uveal melanomas and pituitary macroadenomas. AS is also in phase I-II trials for treatment of breast, colorectal and non-small cell lung cancers. Similarly, a clinical trial in 120 patients with advanced non-small cell lung cancer has shown that artesunate in combination with a chemotherapy regimen of vinorelbine and cisplatin elevated 1-year survival rate by 13% with a significant improvement in disease control and time to progression. No additional AS-related side effects were reported (Zhang et al., 2008).
- Phase I study of oral AS to treat colorectal cancer (Completed)
The primary objective of this study was to determine the effects of oral AS in inducing apoptosis in patients awaiting surgical treatment of colorectal adenocarcinoma. The secondary objective of this study was to establish the tolerability of oral AS for the treatment of colorectal cancer. Subjects were randomized to receive either 200 mg AS or placebo orally once daily for 14 days while awaiting surgery for definitive surgical treatment of colorectal adenocarcinoma. A significant difference in the proportion of colorectal adenocarcinoma cells exhibiting apoptosis was noted between the two treatment groups (placebo and AS), assessed at the time of surgery after two weeks of drug treatment. No result was publicly issued (Protocol Number: ISRCTN05203252, 2008. http://www.controlled-trials.com/ISRCTN05203252/).
- Phase II study of AS treatment as an adjunct to treat non-small cell lung cancer (Completed)
This study was designed to compare the efficacy and toxicity of AS treatment combined with NP (a chemotherapy regimen of vinorelbine and cisplatin) and NP alone in the treatment of advanced non-small cell lung cancer (NSCLC). One hundred and twenty cases of advanced NSCLC were randomly divided into an NP chemotherapy group and a combined AS with NP therapy group. Patients in the control group were treated with the NP regimen of vinorelbine and cisplatin. Patients in the trial group were treated with the NP regimen supplemented with intravenous AS injections (120 mg, once-a-day intravenous injection, from the 1st day to 8th day, for 8 days). At least two 21-day-cycles of treatment were performed. There were no significant differences in the short-term survival rates, mean survival times and the 1-year survival rates between the trial group and the control group, which were 44 weeks and 45 weeks, respectively. The disease controlled rate of the trial group (88.2%) was significantly higher than that of the control group (72.7%) (P < 0.05), and the trial group’s time to disease progression (24 weeks) was significantly longer than that of the control group (20 weeks). No significant difference was found in toxicity between the two treatment groups. Therefore. AS combined with NP can increase the disease controlled rate and prolong the time to progression of patients with advanced NSCLC without significant side effects (Zhang et al., 2008).
- Phase I study with metastatic breast cancer (Completed)
The purpose of this study was to evaluate the tolerability of an adjunctive therapy with AS for a period of 4 weeks in patients over the age of 18 years with advanced metastatic breast cancer, which was defined as a histologically or cytologically confirmed. Women of childbearing potential were tested to rule out pregnancy prior to their treatment. Relevant neurological symptoms, adverse events, and the relation between adverse events and the use of AS, as an adjunct, saliva cortisol profile, overall response rate, clinical benefit, and assessment of patients’ expectations will be monitored as study endpoints. No result of this study has yet been publicly issued (Protocol Number: NCT00764036, 2011, http://www.cancer.gov/clinicaltrials/search/view/print?cdrid=616937&version=HealthProfessional).
3.3.2. Treatment reports of ARTs used to treat cancers
AS was successfully used in the treatment of laryngeal squamous cell carcinoma where treated patients showed a substantial reduction in tumor size (by 70%) after two months of treatment (Singh and Verma, 2002). Furthermore, AS used in combination with standard chemotherapy increased survival and substantially reduced metastasis in patients with malignant skin cancer (Berger et al., 2005). Another report describes a beneficial improvement in a patient with pituitary macroadenoma who was treated with artemether for 12 months (Singh and Panwar, 2006). Other cases describing the use of ARTs for treatment of cancer have been reported in the Cancer Smart Bomb Part I and II study (White, 2002)
- Metastatic uveal melanomas treated with AS
Berger et al. reported on the first long-term treatment of two cancer patients with AS in combination with standard chemotherapy. These patients with metastatic uveal melanoma were treated on a compassionate-use basis, after standard chemotherapy alone was ineffective in stopping tumor growth. The therapy regimen was well tolerated with no additional side effects other than those caused by standard chemotherapy alone. One patient experienced a temporary response after the addition of AS to Fotemustine while the disease was progressing under therapy with Fotemustine alone. The second patient first experienced a stabilization of the disease after the addition of AS to Dacarbazine, followed by objective regressions of splenic and lung metastases. This patient is still alive 47 months after first diagnosis with stage IV uveal melanoma, a diagnosis with a median survival of 2-5 months, without additional side effects. One patient experienced a temporary response after the addition of AS while the disease was progressing under standard therapy with Fotemustine alone. This patient died after 24 months.
Despite the small number of treated patients, AS may be a promising adjuvant drug for the treatment of melanoma and possibly other tumors in combination with standard chemotherapy. AS is well tolerated, and the lack of serious side effects will facilitate prospective randomized trials in the near future. From in vitro studies already conducted (Efferth et al., 2004b), it is further conceivable that loading tumor cells with bivalent iron, by simply providing Fe2+ in tablet form, might increase the susceptibility of cancer cells to AS treatment. It is tempting to speculate that, in the case of the second patient previously discussed, the addition of Fe2+ had an actual clinical impact and resulted in an improved response to therapy (Berger et al., 2005).
- Laryngeal carcinoma treated with AS
AS injections and tablets were used in one study to treat a laryngeal squamous cell carcinoma patient over a period of nine months. The tumor was significantly reduced in size by 70% after two months of treatment. Overall, AS treatment of the patient was beneficial in prolonging and improving quality of life. Without treatment, laryngeal cancer patients die within an average of 12 months. The patient lived for nearly one year and eight months until his death due to pneumonia.
The observations that the patient regained his voice, appetite, and weight after a short term treatment with AS, and the fact that the tumor was significantly reduced in size without any apparent adverse side effects suggests that AS treatment could be an effective and economical alternative treatment for cancer, especially in cases of late cancer detection where available treatments are limited. Since this case report was published, several patients with different types of cancers have begun treatment with ART and its analogs with promising results. AS therapy has potential to prevent and treat a wide range of cancers given its efficacy, low cost, and due to the common mechanisms of action demonstrated against various cancer cells (Singh and Verma, 2002).
- Pituitary macroadenoma treated with AM
AM, an ART analogue, was used to treat a 75-year old male patient with pituitary macroadenoma. This patient presented with vision, hearing, and locomotion-related problems as a consequence of his disease. AM was administered orally to the patient over a period of 12 months. Although the tumor remained consistent in size, CT scans showed a reduction in tumor density, and clinically, the related symptoms and signs improved significantly as therapy progressed. Overall, the AM treatment was beneficial in improving the patient’s quality of life. AM and other ART analogs appear to have promise for treatment of this type of cancer (Singh and Panwar, 2006).
3.4. Why are not there more trials or more wide-spread use of ARTs?
ARTs are largely non-toxic, with related compounds having been administered to over 2 million patients; both children and adult, world-wide without reports of significant serious side effects, and ARTs are very inexpensive when compared to conventional cancer drugs. The final results of current clinical trials utilizing ARTs as therapy or adjunct against a wide variety of cancers have not yet been published although initial findings released suggest positive results. How positive or efficacious these results are remains to be seen until full and final results are published. One question, however, remains – why are there not more trials or more wide-spread use of ARTs as an off-label cancer treatment?
3.4.1. PK mismatch of ARTs in cancer therapy
ARTs as angiogenesis inhibitors are unique cancer-fighting agents because they tend to inhibit the growth of blood vessels rather than tumor cells. Therefore, the angiogenesis inhibitor therapy does not necessarily kill tumors but instead may prevent tumors from growing. This type of therapy may therefore need to be administered over a long period.
Following oral administration, however, AS and DHA have short mean residence times (MRT) of 1.95 and 2.71 hr, respectively, and ART has a longer MRT of 7.4 hr. Intramuscular AM and arteether have longer MRTs ranging from 13.9 – 42.9 hrs in humans s due to prolonged absorption and accumulation at the injection sites. The shortest MRT (0.90 hr) was found in humans following intravenous injection of AS (Table 2). It is obvious that the different ARTs administered in different regimens have significant differences in pharmacokinetic (PK) characteristics in humans, and only intramuscular ARTs can provide a long period of therapy. Therefore, injectable AM has been recommended as a longer acting compound that may be suitable for cancer treatment (White, 2002).
| PK Parameters | AS | AS | DHA | ART | AM | AE |
| Route of administration | Intravenous | Oral | Oral | Oral | Intramuscular | Intramuscular |
| First loading dosage | 120 mg | 100 mg | 200 mg | 500 mg | 3.2 mg/kg | 4.8 mg/kg |
| Maintaining dosage | Oral 100 mg at 8 hr | 50 mg b.i.d. x 4 | 100 mg x 4 | 250 x 2 x 5 | 1.6mg/kg x 4 | 1.6mg/kg x 5 |
| Total dose | 220 mg and mefloquine** | 500 mg | 600 mg | 3000 mg | 9.6 mg/kg | 12.8 mg/kg |
| Cmax (ng/ml) | 2646 (DHA); 11343(AS) | 1052 (DHA); 198 (AS) | 437.5 | 588.0 | 74.9 | 110.1 |
| Tmax (hr) | 0.13 | 0.75 | 1.4 | 2.4 | 6.0 | 8.2 |
| Tlag (hr) | 0.2 | 0.45 | ||||
| AUC0-24 hr (ng·h/ml) | 2378 (DHA); 1146 (AS) | 1334 (DHA); 210 (AS) | 1329 | 2601 | 1230 | 4702 |
| t1/2 (absorption, hr) | 0.36 (DHA) | 0.67 | 1.21 | 1.88 | 3.2 | |
| t1/2 (elimination, hr) | 0.67 (DHA); 0.05 (AS) | 0.70 (DHA) | 0.85 | 2.3 | 7.83 | 22.7 |
| MRT (hr) | 0.90 (DHA) | 1.95 (DHA) | 2.71 | 7.41 | 13.94 | 42.9 |
Table 2.
Pharmacokinetics (PK) parameters of intravenous artesunate (AS), oral AS, oral dihydroartemisinin (DHA), oral artemisinin (ART), intramuscular artemether (AM) and arteether (AE) in human treatments with uncomplicated and severe/complicated malaria on day 1*.
*The data was fitted with WinNonlin (V5.0) by author. **Oral 750 mg mefloquine at 24 hr after IV injection. PK = pharmacokinetics; PD = pharmacodynamics; MRT = Mean residence time; PC50 = Mean time for parasitemia to fall by half; AUIC = area under inhibitory curve; QHS = Artemisinin; DHA = Dihydroartemisinin; AM = Artemether; AE = Arteether; AS = Artesunic acid; MPC = minimum parasiticidal concentration; IM = intramuscular.
Pharmacokinetics (PK) studies of ARTs show three phases (absorption, distribution, and elimination) of ART drugs in blood following oral, intravenous, or intramuscular administration. After multiple daily administrations, four ARTs showed declining daily drug concentrations (ART, DHA, AS, and AM) which has been shown is believed to be due to an auto-induced metabolism pathway during multiple oral treatments in patients and health subjects (van Agtmael et al., 1999; Ashton et al., 1996; 1998; Khanh et al., 1999; Park et al., 1998). The Cmax and AUC values of ARTs are markedly reduced from one-third to one-seventh on the last dose day compared with the first day. The decrease in drug exposure levels during treatment is not disease-related, since the PK profile of ART drugs on the last day shows a similar decrease to that reported in healthy subjects. Similar time-dependent declines have been reported in animals dosed with oral AM (Classen et al., 1999). One possible explanation for the decrease in plasma concentration-time during treatment is an increase in metabolic capacity due to auto-induction of hepatic drug-metabolizing enzymes.
Similar observations have shown that decreasing absorption of ART derivatives may be a problem for the longer-term use required for treatment of cancer. In treating malaria, the ART derivatives are given for a short four or five day course. In these short treatments, no absorption resistance has been observed to occur. Recent information has come to light that indicates that the intestine builds up resistance to absorbing oral ART compounds very quickly, within several days. Resistance is demonstrated by a >30% drop of the original rate of absorption. Research indicates that this resistance can be overcome very quickly by discontinuing use of the ART compounds for several days to a week; when resumed, their absorption will be at the previous higher level. One study author, Dr. Lai, pointed out that this intestinal resistance and subsequent lowered absorption rate may be the basis of the plateau that many patients reach after treatment with these compounds. After an initial quick response, many patients seem to stabilize without a complete remission (White, 2002).
| Artemisinins | Cancer targets | Clinical studies | Protocols & References |
| Artesunate | Colorectal cancer | Clinical trial, Phase I | ISRCTN05203252, 2011 UK |
| Artesunate | Non-small cell lung cancer Metastatic uveal melanoma Laryngeal carcinoma Metastatic breast cancer | Clinical trial Phase I-II Case report Case report Clinical Trial, Phase I-II | Zhang et al., 2008 CHINA Berger et al., 2005 GERMANY Singh & Verma, 2002 INDIA NCT00764036 2008 GERMANY Verified Feb. 2009 |
| Artemether | Pituitary macroadenoma | Case report | Singh& Panwar 2006 INDIA |
Table 3.
Anti-cancer effects of artemisinin (ART), artesunate (AS), and artemether (AM) in case reports of treatments and clinical trials (Ghantous et al., 2010)
All clinical trials listed here are completed
3.4.2. Possible optimization of clinical trials with ARTs
ARTs are anti-angiogenic agents with a variety of targets that inhibit tumor angiogenesis in two ways: 1) blockade of angiogenic pathways and 2) inhibition of endogenous angiogenesis (Efferth, 2006; 2007; Crespo-Ortiz and Wei, 2012). Cancers produce a variety of angiogenic factors or cytokines to stimulate angiogenesis, which is essential for tumor growth and metastasis (Cao and Liu, 2007). These cancer-derived angiogenic factors include VEGF, fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGFs), angiopoietins (Angs), hepatocyte growth factor (HGF), and insulin-like growth factors (IGFs). The angiogenic signals triggered by these angiogenic factors are mediated by their specific tyrosine kinase receptors (TKRs) expressed in endothelial cells (Nissen et al., 2007; Xue et al., 2008).
ARTs responses seem to be mediated by those angiogenic factors with strong multi-targeted anti-angiogenic potency. However, ART targets cancer cells is cleavage of the endoperoxide bridge by the relatively high concentrations of iron in cancer cells, resulting in generation of free radicals such as reactive oxygen species (ROS) and subsequent oxidative damage as well as iron depletion in the cells. Studies demonstrated that co-administration of holotransferrin and other iron sources with ARTs have been shown to increase the potency of ARTs in killing cancer cells (Lai et al., 2009; Mercer et al., 2011; Zhang et al., 2010). Also, DHA in combination with butyric acid acts synergistically at low dose (Singh and Lai, 2005).
Current combinations with chemotherapy for the treatment of patients with cancer have produced only modest beneficial effects (Cao et al., 2009). Optimization of anti-angiogenic therapy is urgently needed in order to maximize therapeutic efficacy of these drugs. Obviously, defining novel therapeutic targets other than VEGF would be an important approach to increase clinical responses as a majority of cancer patients have been shown to demonstrate intrinsic resistance to anti-VEGF therapy. Given the fact that most tumors produce a broad spectrum of angiogenic factors to stimulate angiogenesis and to sustain the established vasculature, it is not surprising that blockade of a single angiogenic pathway would be insufficient to suppress tumor growth and multitargeted “dirty drugs” would be more effective. In support of this view, anti-angiogenic monotherapy with tyrosine kinase inhibitors such as sunitinib and sorafenib targeting multiple signaling pathways has been shown to result in increased survival in patients treated for metastatic renal cell carcinoma (Escudier et al., 2007; Motzer et al., 2006)
ARTs are delivered to cancer patients by systemic administration, which may lead to a universal impact on healthy vasculatures distributed in multiple tissues and organs (Cao, 2010). In the conventional view of anti-cancer drugs, off-tumor targets would be associated with unwanted adverse effects of drugs. Interestingly, clinical benefits of ARTs have been positively associated with neurotoxicity and embryotoxicity, which have been shown to result from the systemic effects of these drugs (Li et al., 2009). In preclinical tumor models, it has been demonstrated that anti-angiogenic agents administered at a low dose normalize vasculatures in healthy tissues including those fenestrated vasculatures in endocrine organs such as bone marrow, liver and adrenal gland without affecting the tumor vasculature (Xue et al., 2008). Normalization of tumor VEGF-induced vascular tortuosity in non-tumor tissues has been shown to significantly prolong the survival of tumor-bearing mice by improving the cancer associated systemic syndrome. These findings suggest that the off-tumor targets of anti-angiogenic agents such as ARTs may provide clinical benefits to cancer patients. Unfortunately, clinical trials based on improvement of paraneoplastic syndrome and cancer cachexia by ARTs have neither been designed nor reported (Cao, 2011).
3.4.3. Animal model results differ from human cancer patients
Preclinical models for assessment of anti-angiogenic and antitumor activities are xenograft tumor models in mice that carry implanted mouse or human tumors. Although this is a commonly used animal tumor model for studying anti-angiogenic and antitumor effects of different molecules, the relevance of this xenograft model to the clinical setting is far from reality. The subcutaneous implantation site does not usually represent physiologically orthotropic sites where human tumors arise. The tissue site is probably one of the most important issues related to response of tumors to drugs because angiogenic vessels in various tissues may express different receptors that are activated by specific ligands. Selective expression of different subsets of the same ligand receptors exists in different tissues. Differential expression of angiogenic factor receptors in various tissues and organs may lead to distinctive ARTs specific responses.
It is known that angiogenesis occurs at different rates in various aged populations (Rivard et al., 1999). Thus, a difference between human cancers and mouse tumor models is the speed of cancer development. In human patients, spontaneous development of a clinical detectable cancer may take years whereas development of a similar sized mouse tumor may only take weeks (O’Reilly et al., 1994). The differential growth rates between human and mouse tumors may create completely different environments, leading to dissimilar angiogenic profiles and drug responses. Young human or animal subjects are susceptible to angiogenic stimuli by triggering relatively robust angiogenic responses under physiological and pathological settings. In contrast, older human or animal subjects often show delayed or impaired angiogenic responses under the same conditions.
In animal tumor models, the endpoint of any drug study is the effect of the drug on tumor size, whereas in human patients, survival improvements by ARTs are often the clinical endpoints measure. Therapeutic efficacy of anti-angiogenic agents is often assessed as monotherapy in animals whereas the same agents are delivered to cancer patients as combinatorial therapy with chemotoxic drugs. In animal tumor models, delivery of chemotherapeutic drugs alone at the conventional dose levels often produces overwhelming anti-tumor effects and addition of anti-angiogenic agents as an extra component would be difficult to enhance the chemotherapeutic effect. Thus, the anti-angiogenic monotherapy with most available drugs has not demonstrated clinical benefits in cancer patients (Cao, 2009; 2011; Hurwitz et al., 2004).
Unlike humans, inbred experimental homogenous mice represent the same genetic background and tumors are artificially manipulated to grow at the same or at least a similar pace. Unsurprisingly, these genetically identical animals would produce a similar response to the same drug. Indeed, anti-angiogenic monotherapy in mice regardless of whether the tumor implanted is a xenograft or derived from a genetically prone mouse tumor model shows the predicted power of drug tumor suppression. Thus, this type of animal model would not be appropriate for assessment of the therapeutic efficacy of ARTs in human cancer patients. Therefore, the difference in anti-angiogenic profiles between human and mouse cancers in relation to the therapeutic efficacy of drug treatment may well explain the variation in human cancer patient responses to ARTs therapy.
3.4.4. Potential toxicities of ARTs in the cancer therapy
There have been a variety of reasons to believe that anti-angiogenic drugs for clinical use as a cancer treatment may have a number of side effects. First, the generation of new blood vessels is a very complicated, multi-step biological process, and VEGF plays an important role in a variety of biological processes such as hematopoiesis, myelopoiesis and endothelial cell survival. Therefore, anti-angiogenic therapy could cause several toxicities due to these pleiotropic biologic effects. Furthermore, many of the angiogenic inhibitors tested target multiple tyrosine kinases in several different pathways, and thus toxicities may not only arise from the inhibition of one pathway but also possibly from the concomitant inhibition of several pathways. Moreover, many of these biological agents are used or will be used in combination with other cytotoxic agents as a treatment strategy. It is not surprising that there is more toxicity in some studies using combination therapies involving angiogenesis inhibitors than those using single agents. The toxicities associated with administration of angiogenesis inhibitors have been shown to include bleeding, disturbed wound healing, thrombosis, hypertension, hypothyroidism and fatigue, proteinuria and edema, skin toxicity, leukopenia, lymphopenia, and immunomodulation (Wu et al., 2008).
In addition, there are published studies showing potential toxicities associated with the use of ARTs as anti-angiogenic agents. Various animal studies have documented neurotoxicity and embryotoxicity associated with ARTs administration, which has raised the question of whether those toxicities might occur in humans, particularly, in anti-cancer therapy and prevention of metastasis.
Neurotoxicity of ARTs
Studies with laboratory animals have demonstrated neurotoxicity associated with a number of adverse effects including movement disturbances, spasticity, balance deficits, brainstem tissue damage, and even death following administration of some intramuscular doses of oil-soluble AM and arteether, or intragastric water-soluble artelinate. There are significant differences in neurotoxicity observed between rats, dogs and rhesus monkeys after treatment with different ARTs suggesting that the exposure time required to induce neurotoxicity after dosing with ARTs is likely to be longer in humans. Since toxicity is dependent on chemical/drug exposure levels and time (Rangan et al., 1997; Rozman and Doull 2000), the neurotoxicity of ARTs has been demonstrated to occur through continued drug exposure over a longer period of time rather than through an elevated drug exposure level over a shorter period of time (Jorgensen 1980; Li et al., 2002 and 2006; Rozman 1998). Accordingly, the 3-5 days dosing duration currently used in ART antimalarial therapy should be quite safe. Neurotoxicity may be caused in humans, however, with inappropriate dose regimens, and therefore, sustained drug exposure times appear to be the critical factor to assess and prevent neurotoxicity (Li and Hickman, 2011).
The current clinical dose regimens of three-day ART combined therapies (ACTs) for uncomplicated cases of malaria, and the dose regimens recommended for intravenous AS treatments for severe malaria which include a few days of a loading doses may be too short of a drug exposure time to induce neurotoxicity in humans. Also, with regard to acute toxicity, humans appear to be less sensitive than animals (Geyer et al., 1990; Kimbrough 1990), and humans appear to have much better repair capabilities than animals to respond to such toxicity (Culotta and Koshland 1994). TK/TD analysis of neurotoxicity after ART treatment has provided a wealth of data to provide a means of predicting the neurotoxic exposure time of ARTs in humans (Li and Hickman, 2011). Based on this data, we predict the safe dosing duration of ARTs in the neurotoxic exposure time should be longer than 7 days (168 hr). Advances in our knowledge of ART-induced neurotoxicity can help refine the treatment regimens used to treat malaria with ACTs as well as injectable AS products to avoid the risk of neurotoxicity. If the drug exposure time of ARTs administered for anti-cancer therapy occurs over 14 days or even longer anti-cancer, ARTs-induced neurotoxicity may well occur (Li and Hickman, 2011).
Embryotoxicity of ARTs
In animal work, there is clear evidence of ARTs-induced embryo death and some evidence of morphological abnormalities in mice, rats, hamsters, guinea pig, rabbits and monkeys in early pregnancy especially after administration of injectable AS (Li and Weina, 2010b). The mechanisms and the pharmacokinetic profiles that affect reproductive toxicity in animal species are currently understood. These animal studies have shown that only injectable AS (intramuscular, intravenous, or subcutaneous) induces reprotoxicity at a lower dose (0.6-1.0 mg/kg) than the therapeutic dose (2-4 mg/kg) in humans. Other doses in different regimens (oral artemisinins or intramuscular AM) are safe at higher levels (6.1-51.0 mg/kg) than the therapeutic doses used. Orally dosing, the most commonly used route of administration in pregnant women with Artemisinin-based combination therapies (ACTs), has been shown to result in lower peak drug concentrations and shorter exposure times, which is less likely to induce embryotoxicity.
Toxicokinetic and tissue distribution data has shown that the severe embryotoxicity induced by injectable AS is associated with six risk factors: 1) Injectable AS can provide much higher peak concentrations (3–25 fold) than oral ARTs or intramuscular AM when administered to animals. In vitro results have shown that the drug exposure level and time are important factors required for induction of embryotoxicity (Longo et al., 2006a; 2006b; 2008). In vivo studies have shown that the drug exposure level, however, is more important than the drug exposure time as AS and DHA both have been shown to have very short half–lives (< 1 h) when administered to various animal species. 2) AS is completely converted to DHA, and therefore, AS serves as a prodrug of DHA. DHA has been shown to be more effective than AS in inhibition of angiogenesis and vasculogenesis in vitro (Chen et al., 2004a; White et al., 2006). 3) Among the ARTs, AS has been shown to have the highest conversion rate to DHA. The conversion rate of AS to DHA was shown to range from 38.2–72.7% while of the conversion rate of AM and AE to DHA ranges from 12.4–14.2%. 4) The conversion rate of AS to DHA was significantly increased in pregnant animals than in non–pregnant rats following multiple injections. 5) The buildup of high peak concentrations of AS and DHA in the plasma of pregnant rats was significantly higher than those of non–pregnant animals after repeated dosing. 6) Injectable AS administration results in a higher distribution of AS and DHA in the tissues of feto–placental units in pregnant animals after multiple administrations (Li et al., 2008).
It is not clear how these findings from animals translate to human patients treated for malaria in with a 3-5 day treatment regimen (WHO, 2006b; Wang, 1989). Data from limited clinical trials in pregnant women (1837 cases) exposed to ART compounds and ACTs, including a small number (176 cases) in the first trimester, have not shown an increase in the rates of abortion or stillbirth; they have also not shown evidence of abnormalities. Since more than 99% of pregnant patients have been treated with oral ARTs or intramuscular AM in the previously referenced trials, the lack of sensitivity and enhanced repair capabilities of humans to respond to ARTs induced embryotoxicity may explain the lack of embryotoxicity observed.
The possible embryotoxicity associated with ARTs therapy should be avoided by limiting exposure of pregnant women in the first trimester which is the critical period for induction of embryo damage and resorption. In addition, to protect pregnant women from embryotoxicity associated with ARTs treatment, injectable AS should be used very cautiously. There is agreement that ART derivatives should not be withheld at any stage of pregnancy, in cases of severe and complicated malaria, if the life of the mother is at risk. It is believed that oral ARTs regimens are much safer than parenteral administrations in pregnant patients. When relating the animal and human toxicity associated with ARTs administration, there are differences in sensitivity, the timing of the most vulnerable period of the embryo to ARTs administration, and the different pharmacokinetic profiles between animals and humans which may possibly provide a greater margin of safety for the use of ARTs by pregnant women.
In accordance with WHO recommendations and the new research described above, the two major issues for considering ART drug use in a program for prevention or management of malaria in pregnant women are safety and efficacy (WHO, 2006a). First, the exposure to injectable AS should be very limited, during the early sensitive period (GD 15 to week 6 in humans), which is the likely critical phase for induction of embryo damage. This is essentially the same recommendation that the WHO has provided where ARTs should not be used in the first trimester of pregnancy in women. Secondly, in uncomplicated malaria WHO recommends that the oral ARTs, including ACTs, should only be used in the second and third trimester when other treatments are considered unsuitable? We feel that oral regimens could be used to treat pregnant women in all trimesters, however, when other treatments are considered unavailable, because the common oral ARTs regimens utilized provide a lower peak concentration and short exposure time, and that can make these ARTs combination drugs safer for use in pregnant women than intravenous or intramuscular injection of AS. Therefore, this policy should also suitable in anti-cancer therapy and prevention (Li and Weina, 2011).
4. Therapeutic implications of new and alternative mechanisms of anti-angiogenesis
Until recently, normal and abnormal processes of angiogenesis were considered to be based on a limited number of known mechanisms. Recent advances have been made in identifying a number of novel alternate processes involved in angiogenesis. If these new findings of alternate mechanisms are confirmed, cancer therapy strategies may also be affected
4.1. New signaling molecules and pathways that influence the angiogenic response
The first generation of clinically useful anti-angiogenic agents including ARTs focused on VEGF and targets in the VEGF pathway. VEGF and its receptors represent one of the best-validated signaling pathways in angiogenesis (Ferrara et al., 2003), and the current FDA approved anti-angiogenic agents inhibit the VEGF pathway (Ferrara, 2010). The strengths and limitations of this therapeutics are now clear. Some tumors do not respond to VEGF-directed therapies de novo, and others become non-responsive or resistant over time by switching to other angiogenic pathways. The next generation of angiogenesis-directed therapeutics will expand the field beyond the VEGF pathway and become more disease selective. New signaling molecules and pathways, including new VEGF-independent cancer angiogenesis pathways, have been recently reported (Teicher, 2011):
- Over-expression of VEGF results in increased angiogenesis in normal and pathological conditions. The existence of an alternative splicing site at the 3’untranslated region of VEGF mRNA results in the expression of isoforms with a C-terminal region which are down-regulated in tumors and may have differential inhibitory effects. This suggests that control of splicing can be an important regulatory mechanism of angiogenesis in cancer cells (Biselli-Chicote et al., 2012).
- The VEGF family includes VEGF-A, -B, -C, -D, -E factors and the placenta growth factor (PIGF). The most studied and best characterized member of the VEGF family is VEGF-A or VEGF, which is secreted by tumors- and plays an important role in both normal and tumor-associated angiogenesis. The biologic effect of VEGF-A is exerted through interaction with cell surface receptors that include VEGF receptor-1 (VEGFR-1, flt-1) and VEGF receptor-2 (VEGFR-2, KDR/flk-1), which are selectively located on vascular endothelium and are up-regulated during angiogenesis, and VEGFR-3, a lymphatic growth factor. The role of VEGFR-1 seems to be complex, and studies indicate that VEGFR-1 may negatively regulate angiogenesis, although it has also been shown that it contributes to vascular sprouting and metastasis. The VEGF-A–VEGFR-2 interaction also plays a crucial role in angiogenesis, through the coordinated signaling of endothelial cell proliferation, migration and recruitment of endothelial cell progenitor cells. VEGF-B has recently been found to be largely necessary for vascular survival rather than angiogenesis (Ferrara, 2009; 2010; Zhang et al., 2009).
- Placental growth factor (PlGF), a member of the VEGF family of growth factors, is induced as tumors lose responsiveness to VEGF-directed therapies (Van de Veire et al., 2010). PlGF was first described, crystallized and identified as a ligand for VEGFR1 in the early 1990s (Ribatti, 2008). The functional biology of PlGF is still being explored. PlGF appears to have direct effects on some malignant cells and has been shown to increase cell proliferation and migration (Chen et al., 2009d).
- Angiopoietins (Angs) are another family of endothelial cell-specific molecules that bind Tie receptors, and they play an important role in vessel maintenance, growth and stabilization. There are four types of angiopoietins known: Ang-1, -2, -3 and -4. Tie1 mRNA is highly expressed in embryonic vascular endothelium, angioblasts, endocardium, and lung capillaries while it is weekly expressed in the endocardium of adults The Tie2 receptor takes part in vessel maturation by transducing survival signals for endothelial cells. Ang-1 acts as an agonist promoting vessel stabilization in a paracrinal manner, whileAng-2 is an autocrine antagonist inducing vascular destabilization at high concentrations. Ang-2 has been found to be dramatically increased during vascular remodeling, and it has been implicated in tumor-associated angiogenesis and tumor progression. It has been found that VEGF also activates the Tie2 receptor (Makrilia et al., 2009).
- The Notch signaling pathway is critical for many developmental processes including physiologic angiogenesis. The Notch pathway has also been shown to have a key role in tumor angiogenesis. Preclinical and clinical studies of various anti-angiogenic combinations suggests that the mechanism associated with poor efficacy may involve tumor resistance and recurrence, which has led to the search for alternative angiogenic treatment strategies. Significant progress has been made in shedding light on the complex mechanisms by which Notch signaling can influence tumor growth by disrupting vasculature in an array of tumor models (Ridgway et al., 2006). The Notch pathway is being investigated as a target for anti-angiogenesis treatment. The VEGF and Notch pathways interact and intersect such that the VEGF pathway stimulates angiogenesis while the Notch pathway helps to guide cell fate decisions that appropriately shape activation (Li and Harris, 2009; Garcia and Kandel, 2012).Delta-like ligand 4 (Dll4) is a key endothelial Notch ligand. The Notch pathway and the VEFG pathway interrelate via the interaction between Dll4 and VEGF. This cross-talk occurs through VEGF-induced upregulation of Dll4 and Dll4 downregulation of the VEGFR signaling. Both pathways are essential for normal angiogenesis, and blockade of one may produce compensatory changes in the other. Dll4–Notch signaling has sparked high interest in exploring molecular targets in these interconnected pathways for cancer therapy (Oon and Harris, 2011)
- Fibroblast growth factors (FGFs) in signaling pathways are a family of heparin-binding proteins required for the development and differentiation of various organs from the early stages of embryogenesis. Acidic and basic fibroblast growth factors (aFGF or FGF1 and bFGF or FGF2 respectively) are described as inducers of angiogenesis. FGFs stimulate endothelial cell proliferation and migration, as well as production of collagenase and plasminogen activator. FGFs induce sprouting of blood vessels in vivo in the chick chorioallantoic membrane and cornea, thus supporting their role in angiogenesis (Makrilia et al., 2009). In addition, the HGF/c-Met pathway is upregulated in some tumors as an alternate angiogenic pathway. The HGF/c-Met tyrosine kinase signaling pathway is upregulated in many cancers resulting in invasive growth consisting of physiological processes including proliferation, invasion and angiogenesis (Eder et al., 2009).
- The CXCL12 (SDF-1)/CXCR4 pathway represents a stromal chemokine axis involved in tumor angiogenesis. CXCR2 is a G-protein coupled receptor with several ligands including interleukin-8 and other angiogenic cytokines and may represent a useful target for anti-angiogenic agents. The CXCL12/CXCR4 axis is involved in tumor progression, angiogenesis, metastasis and survival (Teicher and Fricker, 2010).
- Sphingosine-1-phosphate (S-1-P) is a bioactive lipid that regulates many cellular and physiological processes including cell proliferation, survival, motility, angiogenesis, vascular maturation, immunity and lymphocyte trafficking. Sphingosine-1-phosphate can be neutralized with a monoclonal antibody. Anti-S-1-P antibodies are under investigation as an anti-angiogenic agent. (Hait et al., 2009).
- Several small molecules and antibodies targeting additional pro-angiogenic cell surface molecules are under investigation as anti-angiogenic agents. Tumor necrosis factor-a (TNF-α), transforming growth factor-α (TGF-α), epidermal growth factor (EGF), colony-stimulating factors (CSFs) and others have been implicated in the process of angiogenesis. Several multi-targeted kinase inhibitors each with a unique pattern of inhibitory potency are in clinical trials with a focus on anti-angiogenic activity. Matrix metalloproteinases (MMPs) are a family of enzymes that cleave the extracellular matrix, a process which is considered important for the formation of new blood vessels. Inhibition of MMPs activity seems to be a crucial step in the process of vessel stabilization during the resolution phase of angiogenesis, since uncontrolled proteolysis results in regression of newly formed vessels (Makrilia et al., 2009).
4.2. Potential targets in angiogenesis and angioprevention
Angiogenesis is an essential process in tumor growth, and new basic science research findings in angiogenesis have had considerable impact on cancer therapy research, as the survival and proliferation of cancer is fundamentally dependent on angiogenesis,. In past years, numerous anti-angiogenic agents were developed, and some of them have been applied clinically. Angiogenesis is a complex and multistep process, however, and the intertwining of interrelated angiogenesis pathways is still not completely understood. Discoveries of new and alternative angiogenesis signaling molecules and pathways, combined with studies on the major signaling proteins and pathways related to tumor angiogenesis, have led to new drug development research to target tumor angiogenesis.
Similarly, angiopreventive strategies may involve various targets including angiogenic molecules from tumors cells, inflammatory system and their respective receptors on endothelial cells such as VEGF, PDGF, FGF and their receptors, angiopoietin (Ang) family, endothelial cells, matrix metalloproteinases (MMPs), cyclooxygenases (COXs), lipoxygenases (LOXs) etc. Inflammation, for example, has been shown to be one of the most important processes in mediating angiogenesis, and may be a valid target for mediating anti-angiogenic therapies. Accordingly, long-term angiostasis treatments will likely be an important element in preventing metastasis of tumors that have been treated and are in remission. Emphasis should be placed on screening and identification of non-toxic anti-angiogenic molecules or compounds and their further evaluation in clinical trials to discover the most efficacious anti-angiogenic treatments for cancer therapy.
VEGF-A (VEGF)
VEGF has a number of different gene family members including VEGF-A, -B, -C, -D, and -E and placental growth factor (PlGF). Among them, VEGF-A (or VEGF) has been the most well-characterized and is considered a key angiogenic factor with various splicing variants such as VEGF-A125, -A145, -A165, -A183, -A189, and -A206. VEGF-A is indispensable during embryonic vascular development, and even the loss of a single VEGF-A allele in mice has been shown to result in embryonic lethality due to defective vasculature. Hypoxia, often seen in the center of tumors, strongly up-regulates VEGF-A expression via increased production of hypoxia inducible factor (HIF). Under normal conditions, HIF is ubiquitinated and degenerated by binding to von Hippel-Lindau (VHL) proteins, but, in under hypoxic conditions, HIF cannot bind to VHL, resulting in increased active HIF. HIF acts as a transcriptional activator by mediating transcription at the HIF-1 binding site, the hypoxia response element (HRE), and by enhancing transcription of many pro-angiogenic genes including VEGF-A gene (Ichihara et al., 2011).
VEGF Receptors (VEGFR)
VEGF family members bind to VEGFR (VEGFR-1, VEGFR-2, and VEGFR-3), and VEGF-A binds to VEGFR-1 and VEGFR-2. Although the affinity of VEGF-A to VEGFR-1 is 10-fold higher than it’s binding to VEGFR-2, VEGF-A signaling is mainly mediated by VEGFR-2 because of its intense kinase activity (Olsson et al., 2006). VEGFR-2 signaling in endothelial cells is mediated through downstream cascades such as PI3K/AKT, p38/MAPK, and PLCγ/MAPK, triggering proliferation and migration of endothelial cells, production of proteases, and hyperpermeability of vessels. Currently, researchers agree that VEGF-A/VEGFR-2 signaling is the key pathway for tumor angiogenesis.
VEGF-B and PlGF bind only to VEGFR-1, in contrast to VEGF-A, which binds to both VEGFR-1 and -2. VEGFR-1 signaling has more complex roles in angiogenesis compared with that of VEGFR-2. VEGFR-1 exists as a decoy receptor with high affinity for VEGF-A, and its low kinase activity prevents VEGF-A from binding to VEGFR-2, so VEGFR-1 actually functions as a negative regulator of angiogenesis. In fact, VEGFR-1 tyrosine kinase-deficient mice, with normal ligand binding ability and deficient signal transduction, have been shown to develop normally, which means VEGFR-1 tyrosine kinase activity is not indispensable, at least during development. On the other hand, there is growing evidence that VEGFR-1 can mediate signaling to downstream cascades. VEGFR-1 signaling in bone marrow cells such as macrophage lineage cells has been shown in a subcutaneous injected tumor model to mobilize them to tumor tissues, contributing to angiogenesis and tumor progression (Muramatsu et al., 2010). It has also been reported that VEGFR-1 signaling might be associated with metastasis. Lymphangiogenesis plays an important role in the tumor microenvironment and the formation of new lymphatic blood vessels is considered the first step of tumor metastasis. VEGFR-3 has been shown to induce lymphangiogenesis after binding VEGF-C or –D (Ichihara et al., 2011).
Angiopoietin/Tie2
Ang/Tie2 signaling is an endothelial cell-specific pathway, like VEGF/VEGFR signaling, but it is difficult to target for cancer therapy because of the complex nature of this signaling pathway which will be reviewed in depth later in this chapter. Angiopoietins play an important role in vessel stabilization and maturation, although they cannot directly induce tumor angiogenesis. There are four types of angiopoietins that are known: Ang-1, -2, -3 and -4. Tie1 mRNA is highly expressed in embryonic vascular endothelium, angioblasts and in the endocardium; however, in adult tissues it is expressed strongly in lung capillaries but weakly in the endocardium. The Tie2 receptor takes part in vessel maturation by mediating survival signals for endothelial cells. Ang-1 acts as an agonist promoting vessel stabilization in a paracrinal manner, whereas Ang-2 is an autocrine antagonist inducing vascular destabilization at high concentrations. Ang-2 has been found to be dramatically increased during vascular remodeling and is implicated in tumor-associated angiogenesis and tumor progression. As a further demonstration of the interrelated nature of angiogenic pathways, it has been shown that VEGF also activates the Tie2 receptor (Singh and Milner, 2009; Thomas and Augustin, 2009).
PDGF
The role of platelet-derived growth factor (PDGF) in angiogenesis is not yet fully understood. More recently, PDGF has been found to stimulate angiogenesis in vivo, and experiments with knockout mice have suggested a role for PDGF in the recruitment of pericytes that are needed for the development of capillaries in tumors. PDGF has also been implicated in the vascular aging process. It has been shown that some tumors overcome inhibition of VEGF-mediated angiogenesis by upregulating members of the PDGF family. Epithelial cancers are characterized by paracrine PDGF signaling, whereas autocrine PDGF signaling is implicated in neoplasms such as leukemias, gliomas and sarcomas (Yang et al., 2009). Thus far, four PDGF family members have been identified, PDGF-A, -B, -C, and -D. They form 5 different forms of homodimers and heterodimers, PDGF-AA, -AB, -BB, -CC, and -DD. PDGFs generally act in a paracrine manner in epithelial cancers, while they have been shown to act in an autocrine manner in gliomas, sarcomas, and leukemia. PDGFs are secreted from various cells, and PDGF-A and -C are mainly secreted from epithelial cells, muscle, and neuronal progenitors while PDGF-B is secreted from vascular endothelial cells. PDGF-D secretion is, unfortunately, not well understood (Andrae et al., 2008).
PDGF Receptors (PDGFR)
PDGFs transmit their signal via PDGFRs. When PDGFRs bind PDGFs, PDGFRs dimerize, are autophosphorylated at tyrosine residues in the PDGFR intracellular domain, and the phosphoyrlated PDFGR dimer has been shown to activate downstream pathways, including PI3K, Ras-MAPK, and PLCγ. There are 2 types of PDGFRs, PDGFR-α and PDGFR-β. PDGFRs can form 3 kinds of homodimers and heterodimers, PDGFR-αα, -ββ, and αβ. Considering the five PDGF dimers described above, there could be multiple and complex PDGF/PDGFR pairings. To date, however, there are only three PDGF/PDGFR pairs proven to be functional in vivo, PDGF-AA/PDGFR-αα, PDGF-CC/PDGFR-αα, and PDGF-BB/ PDGFR-ββ. PDGFR-α has been shown to be involved in embryonic development, while PDGFR-β has been shown to be involved in angiogenesis (Cao et al., 2008).
The PDGFR-α-induced pathway is involved in organogenesis such as in alveogenesis, villus morphogenesis, hair morphogenesis, and oligodendrogenesis. In addition, PDGFR-α may indirectly promote angiogenesis by recruiting stromal fibroblast-producing VEGF-A and other pro-angiogenic factors. PDGFR-β is expressed in pericytes but not in endothelial cells, and PCGFR-β signaling is believed to play a role in angiogenesis. Due to PDGFR-β’s expression in pericytes as opposed to endothelial cells, the PDGFR-β signaling pathway does not increase the number of tumor vessels but acts to form mature tumor vessels by recruiting PDGFR-β-expressing pericytes, and, in turn, acting to accelerate tumor growth. Blocking the PDGFR-β pathway inhibits the maturation of blood vessels, eliciting detachment of pericytes and disruption of tumor vessels, while blocking the VEGFR pathway impairs formation of early-stage immature vessels lacking pericyte coverage but does not affect existing mature, large blood vessels well-covered with pericytes (Ichihara et al., 2011).
Delta-like ligand 4 (DLL4)
Delta-like ligand 4 (DLL4) belongs to the Delta/Jagged family of transmembrane ligands that binds to Notch receptors. Delta–Notch signaling has been shown to mediate cell–cell communication and regulates cell fate determination. Delta/Notch signaling is also critically important for proper vascular development. One particular endothelial cell Notch ligand, DLL4, has been shown to be required for regulation of tip cell formation during angiogenesis. Activation of the Delta/Notch signaling pathway has been shown to decrease endothelial tip cell numbers. Conversely, decreased DLL4 signaling increases tip cell formation. Upregulation of DLL4 was also found in tumor vessels. Two groups have demonstrated independently that inhibiting DLL4 leads to tumor growth suppression by deregulating angiogenesis, resulting in increased, but non-functional vessels. Importantly, this strategy is also effective in slowing the growth of tumors that are relatively resistant to anti-VEGF therapy, and DLL4 inhibition also exhibits an additive effect when combined with anti-VEGF therapy to slow the growth of anti-VEGF resistant tumors (Dufraine et al., 2008, Ferrara, 2010).
In fact, not all of the endothelial cells are stimulated, due to a mechanism deciding which endothelial cells should react to angiogenic stimulus and which should not. The DLL4/Notch pathway plays a key role in this mechanism. DLL1, DLL3, DLL4, Jagged1, and Jagged2 bind to the Notch receptor as ligands. Among these ligands related to tumor angiogenesis, DLL4 has been the most intensely investigated, because DLL4 is strongly expressed in tumor vascular endothelial cells but more weakly in normal vascular endothelial cells. DLL4 is a transmembrane ligand, and its expression in tumor vessels is regulated by VEGF-A. VEGF-A up-regulates DLL4 in sprouting endothelial cells (tip cells), and up-regulated DLL4 interacts with Notch in the adjacent endothelial cells (stalk cells). In reverse, the DLL4/Notch pathway down-regulates VEGFR-2 expression in Notch-expressing endothelial cells, resulting in the reduction of VEGF-A-induced sprouting and branching (Lobov et al., 2007). Thus the DLL4/Notch pathway can be considered a negative feedback VEGFR pathway (Ichihara et al., 2011).
Notch
Notch receptors are single-pass transmembrane proteins in a family consisting of Notch1, Notch2, Notch3, and Notch4. The Notch receptor signaling pathway has a characteristic mechanism for signal transduction. After ligand binding, the Notch receptor is cleaved at an extracellular domain by proteases such as ADAM10 or TACE, followed by cleavage at a transmembrane domain by γ-secretase. As a consequence, the Notch intracellular domain translocates to the nucleus and activates the transcription of target genes. Blocking the DLL4/Notch signaling pathway leads to increased angiogenesis, such as the enhancement of tip-cell formation, branching, and vessel density. Paradoxically, blockade of the DLL4/Notch signaling also leads to the inhibition of tumor growth in a variety of tumor models. This is possibly due to an increase in the number of non-functional tumor vessels induced by the DLL4/Notch blockade which in turn results in tumor hypoxia (Scehnet et al., 2007).
FGF1 and FGF2
The fibroblast growth factor (FGF) family has been implicated in neurogenesis, organ development, branching morphogenesis, angiogenesis and various pathologic processes including cancer. Acidic and basic fibroblast growth factors (aFGF or FGF1 and bFGF or FGF2 respectively) have been shown to be inducers of angiogenesis. FGFs stimulate endothelial cell proliferation and migration, as well as production of collagenase and plasminogen activator. FGFs have also been shown to induce sprouting of blood vessels in vivo in the chick chorioallantoic membrane and cornea, thus supporting their role in angiogenesis (Makrilia et al., 2009).
The FGF signaling pathway plays an important role in embryonic organogenesis, and disturbance of this pathway leads to various kinds of developmental defects. In the adult organism, FGF/FGFR signaling is involved in important physiological processes such as the regulation of wound healing and angiogenesis. FGFs are heparin-binding growth factors that are part of a family that includes 23 members, FGF1-23 (Turner and Grose, 2010). Only 18 FGF members work as FGF ligands, because FGF11, 12, 13, and 14 are not functional ligands for FGFR, and the FGF15 gene does not exist in humans. Among these family members, FGF1 and FGF2 have been shown to possess a potent pro-angiogenic effect and they play a role in inducing proliferation and migration of endothelial cells (Daniele et al., 2012).
FGF Receptors (FGFR)
FGFRs belong to a receptor family consisting of FGFR-1, -2, -3, and -4 (Turner and Grose, 2010). FGFRs are expressed in most cells and have various functions, including normal cell growth, differentiation, and angiogenesis., FGFR over expression or mutation has been shown to be associated with a variety of different neoplasms FGFR activation has been shown to induce angiogenesis in both cell cultures and in animal models (Cao et al., 2008; Korc and Friesel, 2009).
TGF-β
The transforming growth factor-β (TGF-β) is thought to have both pro- and anti-angiogenic properties. Low TGF-β levels contribute to a switch in angiogenesis, by up-regulating angiogenic factors and proteinases. On the other hand, high TGF-β levels have been shown to inhibit endothelial cell growth, stimulate smooth muscle cells differentiation and recruitment and promote basement membrane reformation. In cancer cells, multiple mutations in the TGF-β signaling pathway have been described. Elevated TGF-β levels have been shown to induce proliferation of cancer cells, the surrounding stromal cells, immune cells, endothelial cells and smooth muscle cells. High levels of endoglin, which is part of the TGF-β receptor complex, have been detected in cancer patients and are directly correlated with tumor metastasis. More specifically, during the initial stages of tumorigenesis, TGF-β inhibits tumor growth and development by inhibiting cell proliferation and by inducing apoptosis. In later stages, tumor stages become resistant to the tumor suppressioni activity of TGF-β, TGF-β takes on a pro-oncogenic role (Pardali and Dijke, 2009).
Other important tumor angiogenesis targets include:
- The role of integrin ανβ3 in mediating angiogenesis has been shown through its binding of extracellular matrix components and matrix metalloproteinase-2, thus helping to connect new vessels with pre-existing ones, to produce the intra-tumoral vascular network. Ephrin ligands and ephrin receptors play a critical role in blood vessel assembly.
- The role of VE-cadherin in neovascularization has been shown in a number of studies.
- Cadherins have been shown to establish endothelial cell junctional stability in the vessel wall and enhance endothelial cell survival by promoting the transmission of the anti-apoptotic signal of VEGFs.
- Cyclooxygenase-2, an enzyme known to regulate cellular processes such as apoptosis, also has been shown to have an angiogenic effect via thromboxane-A2.
- The fibrinolytic system is another angiogenesis target, and the activation of this system depends on the conversion of plasminogen to plasmin by the tissue-type plasminogen activator (tPA) and the urokinase-type plasminogen activator (uPA).
- Matrix metalloproteinases (MMPs) are a family of enzymes that cleave the extracellular matrix, a process which is considered important for the formation of new blood vessels. Inhibition of the activity of MMPs seems to be a crucial step in the process of vessel stabilization during the resolution phase of angiogenesis, as uncontrolled proteolysis results in regression of newly formed vessels.
- The hypoxia-inducible factors (HIFs) mediate transcriptional responses to localized hypoxia in normal tissues and in cancers, and HIFs have been shown to promote tumor progression by altering cellular metabolism and stimulating angiogenesis. Under conditions of abundant oxygen (N8–10%), HIF-α proteins are translated, but the proteins are rapidly degraded. Stabilization of HIF proteins in hypoxic cancer cells is thought to promote tumor progression, largely by inducing the localized expression of specific target genes encoding VEGF, glycolytic enzymes (PGK, ALDA), glucose transporters (GLUT1) and proteins regulating motility (lysl oxidase) and metastasis (CXCR4, E-cadherin). (Makrilia et al., 2009)
4.3. Vascular normalization in anti-angiogenic cancer therapy
Normal vasculature comprises organized layers of endothelial cells (ECs) and pericytes. There is evidence for paracrine signaling between ECs and specialized organ-specific cells; hence, there is some variation in the structure and function of blood vessels depending on their anatomic location. Pericyte-EC crosstalk facilitates vascular growth and homeostasis, and once vessels mature, these cells become dormant. Blood vessel proliferation is an essential physiological process, and vessel sprouting is one of the major mechanisms of expansion in the network of vessels in growing tumors through filopodia and endothelial stalk cells.
Unlike blood vessels in normal tissue, the tumor-associated vasculature is irregular and unstable, probably due to the over-production of pro-angiogenic proteins such as VEGF. Tumor vessels are distinct in several respects relative to normal vasculature as they are disorganized and tortuous and their spatial distribution is significantly heterogeneous, resulting in uneven drug distribution in tumors. Tumor vessels do not follow the hierarchy of arterioles, capillaries and venules, and tumor vessels are leakier than normal vessels since tumor-associated endothelial cells are widened and loosely connected. Recent studies suggest that tumor ECs have cytogenetic abnormalities including aneuploidy, multiple chromosomes, and multiple centrosomes, raising the possibility that such instability may contribute to resistance to anti-angiogenic therapies. Tumor-associated blood vessels are excessively branched and hemorrhagic, and blood flow through these malformed vessels is often chaotic and may impede delivery of chemotherapy to the tumor itself (Ferrara, 2010; Gordon et al., 2010).
A new therapeutic strategy targeting tumor vasculature has gained increased attention in the scientific community. This method involves targeting abnormal tumor vessel function by inducing vessel normalization. It is well known that tumor blood vessels are highly abnormal in structure and function, characterized by a tortuous, chaotic, and irregular branching network. In the tumor vasculature, ECs are highly activated, lose their polarity and alignment, and detach from the basement membrane, all resulting in a leaky, fenestrated network that facilitates bleeding and increases interstitial fluid pressure. Apart from the ECs, the entire vessel wall, including the basement membrane and the covering pericytes, becomes abnormal in most tumors. Tumor ECs are typically covered with fewer and more abnormal pericytes, and their associated basement membrane is only loosely associated and inhomogeneous in structure. It is suspected that this abnormal vasculature impedes the distribution of chemotherapy and oxygen. Traditional anti-angiogenic therapy aims to maximally inhibit angiogenesis and to prune existing tumor vessels, however, this strategy can also increase the risk of aggravating hypoxia and enhancing tumor cell invasiveness (Carmeliet and Jain, 2011).
Recent genetic and pharmacological studies have revealed that targeting abnormal tumor vessel function by the induction of vessel normalization can offer alternative options for anti-angiogenic therapy. Vessel normalization can be achieved by several different approaches, including blockade of VEGF, genetic modulation of the oxygen sensors prolyl hydroxylase domain containing protein 2 (PHD2), targeting of mechanisms that affect pericyte coverage and vessel maturation, and targeting myeloid cells via blockade or genetic loss of PlGF. Vessel normalization could provide a means to increase the responsiveness to chemotherapy, immunotherapy, or radiation, and may contribute to restricting tumor dissemination (Rolny et al., 2011; Schmidt and Carmeliet, 2011).
One recent study demonstrated that boron targeting of the largest possible proportion of tumor cells contributes to the success of boron neutron capture therapy (BNCT), and tumor blood vessel normalization improves the delivery of boron to the tumor. In this study, blood vessel normalization was induced by administering two doses of thalidomide (Th) in tumor-bearing hamsters on two consecutive days. The effect of blood vessel normalization to enhance the efficacy of boronophenylalanine (BPA) administration was assessed through in vivo BNCT studies at the RA-3 Nuclear Reactor utilizing tumor-bearing hamsters. Overall tumor control at 28 days post-treatment was significantly higher for Th+ BPA-BNCT than for Th- BPA-BNCT with a tumor volume reduction of 84 ± 3% in the Th+ BPA-BNCT group compared to 67 ± 5% in the Th- BPA-BNCT group. Pretreatment with thalidomide enhanced the therapeutic efficacy of BNCT and reduced precancerous tissue toxicity (Molinari et al., 2012). Some studies confirmed, however, that antibodies to VEGF in combination with chemotherapeutic agents produce synergistic cytotoxicity in a range of cancers. Research data shows that the process of normalization of tumor blood vessel structure is not always beneficial. In the case of cerebral tumors, for example, the process of tumor vessel normalization may induce a re-establishment of the low permeability characteristics of normal brain microvasculature, preventing the delivery of chemotherapeutics (Ribatti, 2011).
Despite having an abundant number of vessels, tumors are usually hypoxic and nutrient-deprived because their vessels malfunction. Such abnormal milieu can fuel disease progression and resistance to treatment. Traditional anti-angiogenesis strategies attempt to reduce the tumor vascular supply, but their success is restricted by insufficient efficacy or development of resistance. Preclinical and initial clinical evidence have shown that normalization of tumor vascular abnormalities is emerging as a complementary therapeutic paradigm for cancer therapy and other vascular disorders, which affect more than half a billion people worldwide. Clearly, additional randomized prospective multi-centered trials should be conducted in larger patient populations to confirm these initial clinical data. In addition, critical questions regarding whether vessel normalizing agents can improve tumor oxygenation and drug delivery in human cancers remain to be answered (Carmeliet and Jain, 2011).
4.4. New vascularization/angiogenesis mechanisms in cancer therapy
Before discussing the different ways a tumor is vascularized, we should emphasize that these mechanisms are not mutually exclusive. In fact, in most cases, angiogenesis and neovascularization mechanisms are interlinked, being involved concurrently in physiological as well as in pathological angiogenesis. Although the molecular regulation of endothelial sprouting has been extensively studied and reviewed in the literature, the morphogenic and molecular events associated with alternative cancer vascularization mechanisms are not nearly as well understood. Cancer cells are not generally controlled by normal regulatory mechanisms, but tumor growth is highly dependent on the supply of oxygen, nutrients, and host-derived regulators. It is now established that tumor vasculature is not necessarily derived from endothelial cell sprouting. Cancer tissue can acquire vasculature by a variety of mechanisms to include co-opting pre-existing vessels, intussusceptive microvascular growth, postnatal vasculogenesis, glomeruloid angiogenesis, or vasculogenic mimicry. The best-known molecular pathway driving tumor vascularization is the hypoxia-adaptation mechanism. Other pathways involving a broad and diverse spectrum of genetic aberrations, however, are associated with the development of the “angiogenic phenotype.” Based on this knowledge, novel forms of antivascular modalities have been developed in the past decade.
When applying these targeted therapies, the stage of tumor progression, the type of vascularization of the given cancer tissue, and the molecular machinery behind the vascularization process all need to be considered. A further challenge is finding the most appropriate combinations of antivascular therapies and standard radio- and chemotherapies. The most promising therapeutic plan of action will involve the integration of recent discoveries in this field into a rational strategy to for developing effective clinical modalities using antivascular therapy for cancer (Döme et al., 2007).
Neovascularization is essential for tumor growth and metastasis. An adequate vasculature feeds tumor growth and enhances the potential of metastasis. For many years, tumor vessels were thought to be lined exclusively by endothelial cells (ECs). Therapeutic benefits from promising anti-angiogenic strategies targeting genetically stable ECs, however, are frequently limited by the development of resistance, implying an oversimplified view of tumor vasculature. Recently, great advances in our understanding of cancer vascularization have emerged with several novel mechanisms proposed. In fact, the latest studies of the most lethal ovarian cancers characterized by widespread metastases within the peritoneal cavity have revealed that in addition to ECs, other cells, including bone marrow-derived and plastic tumor cells, contribute to tumor vascularization There are two proposed mechanisms by which tumor-infiltrating bone marrow-derived cells might participate in tumor angiogenesis: (1) direct incorporation in the tumor vasculature and (2) as a source of angiogenic factors such as VEGF-A and MMP-9, which may in turn increase the bioavailability of angiogenic factors.
Current anti-angiogenic therapies have been designed on the assumption that endothelial cells forming the tumor vasculature exhibit genetic stability. Recent studies demonstrate that this is not the case. Tumor endothelial cells possess a distinct phenotype, differing from normal endothelial cells at both the molecular and functional levels. This finding challenges the concept that tumor angiogenesis exclusively depends on normal endothelial cell recruitment from the surrounding vascular network. Indeed, recent data suggest alternative strategies for tumor vascularization, and it has been reported that tumor vessels may be derived from an intratumor embryonic-like vasculogenesis. This condition might be due to differentiation of normal stem and progenitor cells of either hematopoietic origin or cells resident in tissues. Cancer stem cells may also participate in tumor vasculogenesis by virtue of their stem and progenitor cell properties (Bussolati et al., 2011).
During cancer progression, tumors require a blood supply for growth and use the blood supply for metastatic dissemination. It is logical that a stronger ability to form de novo networks and channels providing a stable blood supply may confer a survival advantage for tumors. Ovarian cancers, as discussed previously, can generate tumor vasculature from diverse origins, including EC, EPC, and tumor cells, reflecting a vast capacity for neovascularization, which may help to explain its high malignancy. Thus, anti-angiogenic and vascular targeting strategies against alternative tumor vascularization mechanisms are clearly promising as improved, more efficacious cancer therapies.
The existence of multiple signal pathways and complex regulatory systems in vascular formation means that inhibition of just a single pathway will presumably trigger alternative vascularization mechanisms and additional signal pathways. Therefore, exploring other novel signals in neovascularization is essential for further studies to efficiently target blood vessels in cancer therapy. On the other hand, with the emergence of the concept of normalization of tumor vasculature as a novel form of anti-angiogenic therapy, new vascular signals involved in vascular remodeling are becoming appealing therapeutic strategies to researchers, as a better understanding of these normalization mechanisms may ultimately lead to more effective therapies. Indeed, several novel ligand/receptor pathways are emerging to include: Slit/Robo, semaphoring/plexins, Netrin-1/UNC5B, Delta-like 4/Notch, and others. Interestingly, the first three ligand/receptor pairs are all formerly known to be involved with neuronal axon guidance, implying a possibility that other neural guidance cues may also function as vascular signals. Agents developed from these pathways that control the morphology of the vascular system can induce tumor vascular normalization and, thus, alleviate hypoxia and increase the efficacy of conventional therapies if both are carefully scheduled. Alternatively, blockade of these pathways may result in increased amounts of immature, nonfunctioning vessels, which results in reduced tumor growth, as is the case with blockade of Delta-like 4 (Tang et al., 2009)
In addition, newly published findings suggest that vessels in many non-malignant diseases are also abnormal. Pharmacological approaches used to normalize vessels in cancer can also induce vessel normalization in other angiogenic disorders in animal models and in patients. Moreover, vascular normalization with bevacizumab has provided the first medical treatment to improve hearing in patients with type II neurofibromatosis. Despite treatment advances for coronary and peripheral arterial disease, the burden of these illnesses remains high. To this end, normalization of abnormal vessels has been proposed as a novel strategy to stabilize vulnerable atherosclerotic plaques. One of the challenges for this therapeutic approach is that these strategies stimulate the formation of immature, leaky and disorganized vessels that are poorly perfused, exhibit signs of vessel disorganization and are prone to regression once therapy is halted. Therapeutic normalization of such neovessels would offer the advantage of creating more mature vessels that could deliver oxygen and nutrients more rapidly and efficiently to the ischemic tissue and thereby restore tissue performance (Carmeliet and Jain, 2011).
In contrast with the anti-angiogenic therapy, vascular targeting therapy aims at destroying the existing vasculature of a tumor. Three different classes of vascular targeting therapeutics have been proposed, cytoskeletal disruption, targeted gene delivery, and drug targeting of tumor endothelial cells. The first class, cytoskeletal disruptors, utilizes a combination of combretastatin derivatives which stops blood flow and inhibits tumor growth through the disruption of the tubulin cytoskeleton of endothelial cells which, in turn, leads to vasculature thrombosis. The second class of vascular targeted therapeutics is targeted gene delivery to the neovasculature. This is achieved by using cationic nanoparticles bound to an integrin ανβ3 directed ligand that delivers a mutant gene to tumor vessels. The third class of vascular targeting therapeutics is cationic liposome-based vascular targeting therapy, which relies on a selective propensity for drug delivery to activated tumor endothelial cells. The mechanism on which this targeted drug delivery is based relies on the negative charge associated with angiogenic endothelial cells which in turn attracts cationic liposomes which can actively bind negatively charged angiogenic endothelial cells and deliver cytotoxic drugs (Makrilia et al., 2009).
4.5. Anti-angiogenic gene therapy for cancer
Tumor growth and progression depends on angiogenesis, a process of new blood vessel formation from preexisting vascular endothelial cells. Tumors promote angiogenesis by secreting or activating angiogenic factors that stimulate endothelial proliferation and migration and capillary morphogenesis. The newly formed blood vessels provide nutrients and oxygen to the tumor, increasing its growth. Thus, angiogenesis plays a key role in cancer progression and development of metastases. Anti-angiogenic therapies have demonstrated significant efficacy in some patients, however, several side effects of anti-angiogenic therapy have been noted in the literature. In addition, the cost of several of these therapies is very high and may not affordable for many patients worldwide
VEGF is an important growth factor that promotes angiogenesis and participates in a variety of physiological and pathological processes. Over-expression of VEGF results in increased angiogenesis in normal and pathological conditions. There is significant evidence that alternative splicing of VEGF gene and other genes involved in angiogenesis can regulate the angiogenic process in tumors. Alternative therapies might replace or improve existing ones. In particular, there is a place for pharmaceutical modulation of angiogenic factors affecting pre-mRNA splicing. This can be brought about not only by alteration in the splicing of heparin-binding isoforms of VEGF but also by the relative balance of pro- and anti-angiogenic isoforms. The concept of an angiogenic splicing phenotype, which controls a number of different proteins that can be activated or deactivated by over-expression or activation of key splicing control factors, is an opportunity for intervention that should be explored in greater detail in the near future (Biselli-Chicote et al., 2012).
In eukaryotes genes consist of coding sequences (exons) interspersed with non-coding sequences (introns). The regulation of alternative inclusion/exclusion of exons, or parts of exons, during RNA processing of pre-mRNA into mRNA (alternative splicing) allows a dramatic increase of the protein repertoire versus the gene repertoire. In a number of cases, alternative splicing of mRNA has been shown to generate proteins with distinct, sometimes opposite, functions from a given gene. Angiogenesis is the process of vascularization in physiological conditions, and there are a number of pathologies, including cancer, where angiogenesis favors tumor progression and dissemination of metastasis. In this chapter, we discuss some key examples showing how alternative splicing may induce a switch from anti-angiogenic to pro-angiogenic functions reciprocally. For some of these splicing events, the molecular mechanisms that trigger alternative splicing toward one or the other direction are now becoming known. The emergence of strategies enabling the regulation of alternative splicing opens new routes for anti-angiogenic therapies (Munaut et al., 2010).
In tumors of the nervous system, tumor derived endothelial cells (TECs) may present the same genetic amplification or chromosomal aberrations of the tumor of origin. In human xenografts of renal carcinoma, melanoma, and liposarcoma, murine TECs are aneuploid, bearing alterations similar to those observed in human TECs. This observation remains unexplained. It cannot be ascribed to cell fusion among tumor and endothelial cells, as no human DNA was present in murine TECs. The researchers who conducted this research speculated that the tumor microenvironment may produce factors capable of inducing genetic instability, or loss of tumor suppressors and/or check point activity, resulting in aneuploidy. Altogether, these data suggest two different explanations for the origin of TECs. The first is that they originate from a common progenitor of tumor and endothelial cells targeted by neoplastic transformation; the second is that the effect of the tumor microenvironment leads to genetic instability (Gardlik et al., 2011a).
In addition, the differentiation of cancer stem cells into endothelial cells and the consequent involvement of these cells in tumor vascularization have been recently described in different tumors. The definitive proof that tumor stem cells are bipotent relies on the ability of clones of tumor stem cells to differentiate in vitro and in vivo into both tumor epithelial and endothelial cells (Bussolati et al., 2008; 2009). More recently, the ability of tumor cells to differentiate into endothelial cells has also been reported for cancer stem cells present in neuroblastomas (Alvero et al., 2009). In particular, only a fraction of stem cells, characterized by CD133 and CD144 co-expression (Wang et al., 2010), or in a recent report, by co-expression of Oct4 and tenascin C, shows vasculogenic potential and is selectively localized in the proximity of tumor vessels (Pezzolo et al., 2011). An alternative mechanism of tumor blood perfusion implies the possibility that tumor cells form channels connected to the tumor vasculature, a process defined as “vasculogenic mimicry”. Alternatively, the process of tumor vasculogenic mimicry could be interpreted as being dependent on tumor stem cells (El Hallanti et al., 2010; Yao et al., 2011), as a transitional step in stem cell differentiation toward endothelial cells (Bussolati et al., 2011).
In cancer therapy, recent investigations have focused on using genetically modified bacteria to actually block tumor angiogenesis. Despite recent progress, only a few studies on bacterial tumor therapy have focused on anti-angiogenesis. Bacteria-mediated anti-angiogenic therapy for cancer, however, is an attractive approach given that solid tumors are often characterized by increased vascularization.
The first modern attempts at using bacteria for therapeutic purposes were made more than 40 years ago by showing that bacteria could predominantly replicate in solid tumors. The first indications of this phenomenon, however, date back to the 19th century. These findings remained largely unexplored until the turn of the 20th century, when oncolytic bacteria capable of lysing host cells were first studied by various research groups. The utilization of bacterial systems for therapeutic anti-cancer purposes is further enhanced by genetic modifications, which make them a very promising tool for targeted delivery of genes and their products. Specific advantages of using bacteria for anti-cancer gene therapy include the natural oncolytic potential of some strains/species, direct targeting of tumor tissues and the ease of positive regulation/eradication. The anti-cancer effect of tumor-targeting bacteria can also be achieved after oral administration, which may circumvent the use of intravenous routes of delivery and associated adverse events of intravenous therapy (Chen et al., 2009; Gardlik et al., 2011b).
5. Further development of ARTs as anti-angiogenic cancer agents
Cancer angiogenesis has been confirmed by measurement of high proliferation indices for endothelial cells, not only in rapidly growing animal tumors, but also in human tumors. The rationale for developing anti-angiogenic strategies for cancer therapy was based on the fact that physiological angiogenesis only occurs in a limited number of situations, such as in wound healing and during menstrual cycle. This suggests there is an opportunity for developing highly tumor-specific anti-angiogenic applications which utilize drugs such as the ARTs which have demonstrated anti-angiogenic efficacy with little toxicity.
5.1. Further targeting anti-angiogenesis of ART and its derivatives
Our current knowledge of the anti-cancer mechanism of ARTs is derived from our knowledge of the antimalarial activity of ARTs. The potent anti-cancer activity of ARTs can be attributed to the endoperoxide bond of the ARTs compounds which is shared with the antiparasitic activity of ARTs. In most of the cancers studied, preloading of cancer cells with iron or iron-saturated holotransferrin triggers ART cytotoxicity with an increase in the activity of ARTs. It has been hypothesized that iron-activated ARTs induce damage by release of highly alkylating carbon-centered radicals and radical oxygen species (ROS). Generation of free radicals may play a role in the cell alterations reported in ARTs-treated cancer cells such as enhanced apoptosis, arrest of growth, inhibition of angiogenesis, and DNA damage. In addition, ARTs-sensitive cancer cells have been shown to have down-regulated expression of oxidation enzymes while cancer cells with over-expression of these molecules are more resistant to ARTs therapy. The antineoplastic toxicity of ARTs appears to be also modulated by calcium metabolism, endoplasmic reticulum (ER) stress, and the expression of the translationally controlled tumor protein, TCTP, a binding calcium protein which has been also postulated as a parasite target. Although the expression of the TCTP gen, tctp, was initially correlated with cancer cell responses to ARTs, a functional role for TCTP in the anti-cancer activity of ARTs has yet to be found. As for malaria parasites, the role of sarcoendoplasmic Ca2+ ATPase (SERCA) as a target of ARTs in cancer cells has also been explored (Crespo-Ortiz and Wei, 2012).
ART and its bioactive derivatives elicit their anti-cancer effects by concurrently activating, inhibiting and/or attenuating multiple complementary cell signaling pathways, especially those associated with the VEGF family, based on published data. The precise mechanism of new and alternative actions and other primary targets of ARTs, however, will require further study. In anti-cancer therapy, it has been postulated that ARTs may target organelles such as pathways involving PlGF growth factors (a VEGF subfamily),, angiopoietins, such as the Angs proteins, the Notch signaling pathway, signaling pathways involving fibroblast growth factors (FGFs), and the matrix metalloproteinase (MMPs) family of enzymes (Crespo-Ortiz and Wei, 2012). In a recent study, investigators discovered a panel of genes containing many fundamental regulators of angiogenic regulators, such as VEGF, was found that correlate with the cellular response to AS. These genes govern the stimulation, proliferation and migration of endothelial cells, a fundamental step in vessel formation. The investigators decided to further limit their cluster analysis by including in the cluster analysis only those genes whose mRNA expression correlated with GI50 values of at least four ARTs (Anfosso et al., 2006). Three human genes coding for VEGF (VEGFA, VEGFB, and VEGFC) were discovered in this cluster of ARTs-affected angiogenic regulating genes.. Despite the continuous investigations on new targets, the ART compounds exert common as well as distinct cellular effects depending on the phenotype and tissue origin of the human cancer cells examined. (Firestone and Sundar, 2009).
In addition, most of these studies were based on the consideration that an ideal ARTs as an anti-angiogenic drugs may target different types of tumor, assuming endothelial cells to be similar in different tumor types and genetically stable. The therapeutic efficacy of ARTs, however, was not as successful as expected and endothelial cells acquired drug resistance. This setback is possibly due to the fact that most anti-angiogenic drugs were tested on normal endothelial cells. In light of the involvement of angiogenesis and vasculogenesis in tumor vascularization, it can be speculated that tumor cytotoxic therapies, radiotherapy, and anti-angiogenic drugs, may stimulate vasculogenesis by inducing tumor hypoxia and/or an epithelial mesenchymal transition. Therefore, targeting both angiogenesis and vasculogenesis in tumors may be required to inhibit tumor vascularization, growth, and invasion. In particular, an improved knowledge of the relative contribution of vasculogenesis to tumor vascularization is likely to be critical for development of specific therapeutic strategies (Li et al., 2011).
5.2. Prevention and therapy strategies of ARTs for cancer treatments
Angiogenesis inhibition therapy does not necessarily kill tumors but instead may prevent tumors from growing. Therefore, this type of therapy may need to be administered over a long period of time. Some common components of human diets also act as mild angiogenesis inhibitors and have therefore been proposed for angioprevention, the prevention of metastasis through the inhibition of angiogenesis. Phytochemicals and ART-mediated anti-angiogenic intervention is a growing area of research that may provide an effective cancer prevention strategy. Suppression of pathological angiogenesis by phytochemicals and ARTs could have potential applications in cancer prevention and therapy as well as in other diseases with similar etiology. Chemopreventive phytochemicals are generally non-toxic and hence will produce minimal side effects. In addition, endothelial cells lack induced drug resistance and, therefore, angioprevention could be a preferred strategy for cancer control in comparison to other therapies such as radiotherapy and chemotherapy.
Several anti-angiogenic strategies have been developed to inhibit tumor growth by targeting different components of tumor angiogenesis. Non-toxic natural chemopreventive agents that could be part of the daily human diet have been shown to safely target and inhibit different aspects and components of the process of angiogenesis. ART and its derivatives, and other sesquiterpene lactones, have been shown to have potent anti-angiogenic effects in tumor cells as well as in healthy rat embryos in culture. These observations have many implications in terms of cancer therapy as well as cancer prevention since angiogenesis is a promotional event (Firestone and Sundar, 2009).
Studies have shown that, the upper limit of a tumor mass in the absence of angiogenesis is 1-2 mm, and this size limit is related to the maximum size possible for simple diffusion of nutrients and gases like CO2 and O2. This 1-2 mm tumor size can be maintained by the balance of cell proliferation and apoptosis leading to dormancy of small tumors for many years. Therefore, for tumor growth and the development of vasculature is critical to proceed towards tumor progression and metastasis., Endothelial cells are a preferential target for therapy because they are common to all solid tumors, and endothelial cell proliferation normally occurs only in pathological conditions such as injury or endometrial development. Large number of angiogenic factors, such as VEGF and bFGF. etc., is secreted by tumor cells are required to cause endothelial cell recruitment and proliferation (Ferrara et al., 2003).
These stimuli are constantly present so the differentiation of the tumor endothelium into a mature vessel network is rarely complete, and tumor vessels show an abnormal morphology. The immature vessel network of tumors is a promising anti-angiogenic target for ARTs compounds. The body of knowledge of endothelial cell physiology and tumor angiogenesis obtained through recent research has been crucial to actually understand some of the mechanisms of how ARTs actually exert their anti-angiogenic effects (Efferth, 2005; 2007).Endothelial cells are non-transformed cells, and they should be quite accessible to treat with physiologically achievable concentrations of ARTs (Efferth, 2006; Crespo-Ortiz and Wei, 2012). This therapeutic strategy may involve various targets including angiogenic molecules from tumors cells and inflammatory system (such as neutrophils and macrophages) such as VEGF, bFGF, TNFa, IL-8, etc. and their respective receptors on endothelial cells, endothelial cells itself, matrix metalloproteinases (MMPs), cyclooxygenases (COXs), lipoxygenases (LOXs) etc (see Section 3). Therefore, long-term angiostasis treatment will likely be necessary for cancer prevention and control. This multitargeted anti-angiogenic strategy suggests drug discovery and development should be focused on finding small, non-toxic anti-angiogenic molecules or compounds to be used in a multifaceted cancer control regimen (Firestone and Sundar, 2009).
Large numbers of chemopreventive agents, such as ARTs, have been shown to possess anti-cancer activities in many studies. These agents achieve anti-cancer activities through various mechanisms by targeting different aspects of cancer progression and development. Since angiogenesis is a pre-requisite for the growth of solid tumors, vascular targeting has been explored as a potential strategy to suppress tumor growth and metastasis. In this regard, many phytochemicals or ARTs have been shown to target tumor angiogenesis using in vitro and in vivo model systems (Chen and Cleck, 2009a; Cao et al., 2009; 2011).
Since, angiogenesis is critically important for physiological process such as wound-healing, acute injury healing, and healing of chronic ulceration of the gastrointestinal mucosa, administration of ARTs compounds that inhibit tumor angiogenesis might also suppress physiological angiogenesis and produce critical side effects when dosed over a long period of time. Therefore, anti-angiogenic chemopreventive ARTs and phytochemicals should be studied and analyzed first for their selective targeting of tumor specific angiogenesis to find the most effective anti-tumor combinations (Bhat and Singh, 2008; Crespo-Ortiz and Wei, 2012).
5.3. Combination strategies to enhance efficacy and to prevent resistance of ARTs
Anti-angiogenesis is a cytostatic therapy that is likely to have greatest effect when combined with cytotoxic therapy. It has recently been suggested that anti-angiogenic drugs represent the universal chemosensitizing agents for cancer treatment. There are a number of mechanisms for the observed synergism between anti-angiogenic agents and anti-cancer chemotherapeutic agents that have been proposed 1) the normalization of tumor microvessels by anti-angiogenic therapy which enhances chemotherapeutic drug delivery, 2) prevention of tumor cell repopulation by anti-angiogenic drugs during the break periods after maximum tolerated dose chemotherapy and 3) augmentation of the antivascular effects of chemotherapeutics by anti-angiogenic drugs (Makrilia et al., 2009). There is growing evidence supporting the use of ART and its derivatives in cancer therapy given their potent antiproliferative, antimetastatic and anti-angiogenic activity, which makes them potential anti-cancer drugs. In a combination therapy for cancer, the antineoplastic action of ART may contribute to an independent antitumor activity with no additional side effects. The benefits of combining ARTs with other anti-cancer agents have been investigated showing that the multifactorial activity of ARTs in different pathways may provide synergism and improve overall activity (Liu et al., 2011).
Drug combinations that involve ARTs have been reported in vitro, which show value in this approach, both as a sensitizing agent to chemotherapy in solid tumors (Sieber et al., 2009), and as a synergistic partner with doxorubicin in leukemia (Efferth et al., 2007). Incubation of cancer cells with DHA alone was found to be less effective than in combination with holotransferrin, indicating that intracellular iron plays a role in the cytotoxic effects of DHA (Lai and Singh, 1995). In addition to conventional chemotherapies, ART was also shown to be effective when combined with the immune modulatory drug LEN (Galustian and Dalgleish, 2009). These in vitro studies demonstrated the effects of ARTs on the cell cycle, and these studiesalso demonstrated restoration of cytotoxicity in an ART-resistant cell by adopting a pulsed-schedule of combination treatment.
Many anti-angiogenic and antivascular agents are now in clinical trials for the treatment of cancer. It is conceivable that loading tumor cells with bivalent iron by simply providing Fe2+ in tablet form might increase the susceptibility of cancer cells to the action of AS. In a clinical study of humans with uveal melanoma, one of the patients enrolled was treated with bivalent iron and artesunate, and it is tempting to speculate the addition of Fe2+ had an actual clinical impact and resulted in an improved response to therapy (Berger et al., 2005). Continued research in this area is encouraged by the recent success of a Phase II clinical trial of AS combined with NP chemotherapy in treatment of advanced non-small cell lung cancer. The disease controlled rate of the trial group of AS plus NP chemotherapy (88.2%) was significantly higher than that of the NP chemotherapy alone group (72.7%), and the trial group’s time to progression (24 weeks) was significantly longer than that of the NP chemotherapy alone group (20 weeks). AS combined with NP chemotherapy can increase the short-term survival rate of patients with advanced non-small cell lung cancer and prolong the time to progression without extra side effects (Zhang et al., 2008). The diversity in the targets of ART supports the possibility that it could be used in combination with other agents.
In addition, it has been reported that resistant cancer cell lines become sensitive by adding ART to the conventional treatment (chemosensitization). Interestingly, DHA and AS have exhibited the strongest chemosensitizing/synergistic effects, while other ARTs shows only additive and antagonistic interactions (Singh and Lai, 2005). DHA was shown to synergistically enhance tumor growth inhibition by 45% when administered in combination with gemcitabine, while other ARTs showed only additive effects (Wang et al., 2010). Consistent with this observation, a greater antitumor activity was observed when DHA was used in a combination with cyclosphosphamide in murine Lewis lung carcinoma cell line or in combination with cisplatin in non-small cell lung cancer A549 in mice (Zhou et al., 2010). In rat C6 glioma cells, addition of 1 μM DHA increased the cytotoxic effect of temozolomide, a DNA alkylating agent used in the treatment of brain cancer, by 177%,. Further investigation showed that DHA promotes apoptotic and necrotic activity of temozolomide through ROS generation (Huang et al., 2008).
Recently, an enhancement of the anti-cancer activity of AS was shown in different combination regimens. A striking synergy was achieved in combinations of AS and the immunomodulator drug, lenalidomide (Liu et al., 2011). Overall, this evidence suggests that DHA and AS have remarkable ability to potentiate antitumor agents and to counter tumor resistance. ARTs also have been shown to improve ionizing-based therapies. In the glioma cell line U373MG, DHA treatment was shown to inhibit the radiation- induced expression of GST with concomitant ROS generation. A combination treatment with DHA has been shown to be more effective than radiation or DHA alone (Kim et al., 2006). The adjuvant effect of ARTs in other cancer treatments including hyperbaric oxygen has also been reported (Ohgami et al., 2010).
Current available ARTs in combination with chemotherapy for the treatment of cancer patients have produced only modest beneficial effects (Cao et al., 2009). Optimization of anti-angiogenic therapy is urgently needed in order to maximize therapeutic efficacy of these drugs. Thus, development of a new generation of drugs targeting diverse angiogenic pathways is expected to improve the anticancer benefits of ARTs therapy. In preclinical tumor models, it has been shown that a combination of anti-angiogenic agents with different mechanistic principles yielded a synergistic effect on tumor suppression. Translation of this preclinical finding to patient therapy would suggest acombination of different of anti-angiogenic agents combined with a chemotherapeutic agent will enhance cancer therapy, and such a combination should be considered in future clinical trials (Cao, 2011).
The mechanism(s) underlying the interaction of combinations of anti-angiogenic agents such as the ARTs plus chemotherapeutic treatment, and, indeed, the mechanism of ART action against cancer is still not fully understood, however, many studies in this field of research are ongoing and will guide the basis of further studies and clinical trials (Liu et al., 2011). Scientists investigating the cancer-fighting properties of ARTs have found early evidence that combining it with an existing cancer drug has the potential to make each drug more effective in combination versus when these drugs are used alone. There is currently limited published data exploring the value of ARTs as a combination partner in treatment regimens. These studies have used simple approaches to studying drug–drug interactions, and as a consequence, their conclusions are still open to debate (Liu, 2008).
5.4. Strategies to avoid potential drug toxicities of ARTs
At high concentrations, ARTs appear to be active against cancer in vivo. The use of ARTs at high concentrations or for long drug exposure times, however, has substantial risk of severe toxicities, including embryotoxicity and neurotoxicity. Animal studies have shown that high peak concentrations of AS and DHA can induce embryotoxicity, and the longer exposure times associated with therapy using oil-soluble ARTs, such as AM, will produce fatal neurotoxicity (Li et al., 2007a). To prevent embryotoxicity in pregnant women with malaria, current WHO policy recommendations on the use of ARTs in uncomplicated malaria state that ARTs should be used only in the second and third trimester, limiting the use of ARTS in the first trimester to cases where it is the only effective treatment available (WHO 2006b).
Studies with laboratory animals have demonstrated fatal neurotoxicity associated with intramuscular administration of AM and AE or oral administration of artelinic acid. These effects suggest that the exposure time of ARTs was extended in these studies due to the accumulation of drug in the bloodstream, and this accumulation, in turn, resulted in neurotoxicity. In one study, the drug exposure time with a neurotoxic outcome (neurotoxic exposure time) was evaluated as a predictor of neurotoxicity in vivo (Li and Hickman, 2011). The neurotoxic exposure time represents a total time spent above the lowest observed neurotoxic effect levels (LONEL) in plasma. The dose of AE required to induce minimal neurotoxicity required a 2-3 fold longer exposure time in rhesus monkeys (179.5 hr) than in rats (67.1 hr) and dogs (113.2 hr) when using a daily dose of 6-12.5 mg/kg for 7-28 days, indicating that the safe dosing duration in monkeys should be longer than 7 days under this exposure. Oral artelinic acid treatment required much longer LONEL levels (8-fold longer) than intramuscular AE to induce neurotoxicity, suggesting that water-soluble ARTs appear to be much safer than oil-soluble ARTs. Due to the lower doses (2-4 mg/kg) used with current ARTs and the more rare use of AE in treating humans, the exposure time is much shorter in humans. Therefore, the current regimen of 3-5 days dosing duration should be quite safe. Advances in our knowledge of ART-induced neurotoxicity can help refine the treatment regimens used to treat malaria with oral ARTs as well as injectable AS products to avoid the risk of neurotoxicity. Although the water-soluble ARTs, like AS, appear to be much safer, further study is needed in when employing ARTs as anti-cancer agents (Li and Hickman, 2011).
Thus, rapid elimination of ARTs in oral formulations is safer than slow-release or oil-based intramuscular formulations (Efferth and Kaina, 2010). Remarkably, although ARTs derivatives have been widely used as antimalarials, their toxicity in humans have been shown to be negligible. In cancer therapy, ARTs may have multiple benefits as it can be used in combination with no additional side effects, but also it enhances potency and reduces doses of more toxic anti-cancer partners. Clinical doses used in malaria treatment after ART administration of 2 mg/kg in patients raise plasma concentrations to 2640 ± 1800 μg/L (approximately 6.88 ± 4.69 mM) which can be considered up to 3 orders of magnitude higher than those ART concentrations with antitumor activity (Efferth et al., 2003). It becomes relevant to closely monitor the safety of long-term ARTs-based therapies as severe side effects may be highly unusual but significant. So far, ARTs treatments for as long as 12 months have been reported with no relevant side effects (Berger et al., 2005; Singh and Verma, 2002; Singh and Panwar 2006).
5.5. Strategies to utiliize current and novel ARTs as anti-cancer agents
A number of first generation derivatives of ART have been created (DHA, AS, AM and AE), and other novel compounds have been synthesized as second generation derivatives designed to improve the anti-cancer activities of ART. This second generation ART derivatives have shown remarkable anti-angiogenic effects and cytotoxicity towards tumor cells (D’Alessandro et al., 2007; Krishna et al., 2008). Ether-linked dimers of DHA, for example, have been shown to cause accumulation of tumor cells in the G1 phase of the cell cycle (Morrissey et al., 2010). New growth-inhibitory ART derivatives containing cyan and aryl groups have been shown to cause accumulation of P388 and A549 cells in the G1 phase (Li et al., 2001). Finally, deoxoartemisinyl cyanoarylmethyl ART derivatives with cytotoxic activity have been shown to induce a significant accumulation of L1210 and P388 cells in the G1 phase (Wu et al., 2001)., ART-like endoperoxides have also been synthesized chemically which show greater cytotoxicity towards tumor cells than native ART, which aids in preserving the natural resources of the A. annua plants (Soomro et al., 2011).
As mentioned above, AS is completely converted to DHA and is best described as a prodrug of DHA. DHA has been shown to be more effective than AS in inhibition of angiogenesis and vasculogenesis in vitro (Longo et al., 2006a; 2006b; Chen et al., 2004b; White et al., 2006). In addition, the embryotoxicity and neurotoxicity of ARTs can be reduced by using artemisone, which is a novel derivative of ART that is not metabolized to DHA (D’Alessandro et al., 2007; Schmuck et al., 2009).
Artemisone is a novel amino alkyl ART that has recently entered Phase II clinical trials (D’Alessandro et al., 2007). The compound was rationally designed to have reduced lipophilicity in order to impede transport to the brain and embryo. In addition, the inclusion of a thiomorpholine 1,1-dioxide group at the C10 position blocks the conversion of artemisone to the more lipophilic DHA. This structural modification does not affect anti-parasitic activity but reduces neurotoxicity and embryotoxicity, as assessed in vitro against primary neuronal brain stem cell cultures from fetal rats and in vivo in female rats (Schmuck et al., 2009). The retention of artemisone antimalarial activity infers that chemical activation of the peroxide bridge to a toxic parasiticidal chemical species remains unchanged, but recent literature also suggests that artemisone has a direct cytotoxic activity without activation of the endoperoxide bridge. In fact, two subsequent studies have provided conflicting results concerning the dependence of the pharmacological activity of artemisone on iron-activation of the endoperoxide group.
Interestingly, an in vitro study by D’Alessandro et al, showed that the anti-angiogenic effects of artemisone were reduced compared with DHA, and it was suggested that this reduction may limit the potential of artemisone to cause embryotoxicity mediated by defective angiogenesis and vasculogenesis during embryo development (D’Alessandro et al., 2007). Together these studies suggest that, while artemisone was designed to optimize safety by physicochemical means, the structural changes induced to create artemisone may also affect the intracellular chemical and molecular pathways which underlie toxicity, perhaps via reduced or alternative mechanisms of bio-activation and/or reduced cellular accumulation, when compared with the traditional ARTs. Therefore, artemisone represents an exciting novel compound in which increased anti-parasitic activity is combined with a reduced potential to cause both embryotoxicity and neurotoxicity.
Increased knowledge of the molecular mechanisms of ART-derived drugs and recent synthesis of novel ART derivatives demonstrates that further pharmacokinetic and pharmacodynamic analyses of novel ART derivatives are needed to understand why these compounds differ in efficacy and toxicity. This information will prove useful for the rationale design of more-effective ART-based molecules for use as anti-cancer agents. New derivatives of ARTs may act not only as treatment drugs, but also may have potential as potent cancer preventative agents due to their inhibition of tumor promotion and progression.
Recently, a series of DHA derivatives were synthesized via an aza-Michael addition reaction, and these novel compounds showed a high selectivity index and an IC50 in the nanomolar range against HeLa cells (0.37 μM) (Feng et al., 2000). In another study, a series of deoxoartemisinins and carboxypropyldeoxoartemisinin compounds were synthesized, and the antitumor effects of these compounds were not associated with lipophilicity, as has commonly been assumed, but instead was associated with distinctive boat/chair molecular conformations which facilitated the interaction of these novel compounds with receptors (Lee et al., 2000). In many studies, there has been an emphasis on the nature and stereochemistry of the dimer linker which may influence anti-cancer activity. It has also been shown, however, that the linker by its own is inactive. Morrisey et al. have described that an ARTs dimer exhibits up to 30-fold more activity than ARTs against prostate cancer lines (Morrissey et al., 2010). This dimer selectively exerted both higher cytostatic activity and apoptosis in C4-2 (a cell line derived from LNCaP) and LNCaP cells compared to ARTs (Morrissey et al., 2010). The stereoisomery of the linker may be associated with enhanced anti-cancer activity (Alagbala et al., 2006). In another study, C12 non-acetal dimers and one trimer of deoxoartemisinin showed similar potency to that of the conventional anti-cancer drugs against many cell lines. The linker with one amide or one sulfur-centered 2 ethylene group was essential for potent anti-cancer activity (Jung et al., 2003). The mechanism underlying the antiproliferative action of the ARTs-derived dimers is not clear and requires further study.
Recently, a series of easily synthesized, potent ARTs-like derivatives with anti-cancer activity were created. These endoperoxides exhibit high chemical stability and greater cytotoxicity than AS against cancer cell lines. These compounds also exhibit relevant anti-angiogenic properties as judged by studies in a zebrafish model (Soomro et al., 2011). To overcome the short half-lives of ARTs, novel, longer lasting derivatives will be required. One such example is synthetic trioxolanes, endoperoxide drugs which were created to provide long lasting efficacy against Schistosoma species. ARTs compounds share the endoperoxide bridge structural feature of the trioxolanes, and they have been shown to have prophylactic activity towards the younger developmental stages of Schistosoma but are ineffective as curative agents. The synthetic trioxalane compounds incorporate the endoperoxide “warhead” with enhanced pharmacokinetic properties and exhibit greater efficacy as curative agents against established Schistosoma infections (Xiao et al., 2007). Given that ARTs may be potentially used as anti-cancer drugs and possibly in other parasitic and viral infections, the development of novel endoperoxide compounds with enhanced pharmacokinetic properties and targeted anti-cancer activity is essential. These promising research findings suggest it is possible to identify safer and more effective strategies to treat a range of infections and cancer (Crespo-Ortiz and Wei, 2012).
6. Perspectives and conclusion
ART and its bioactive derivatives are potent anti-cancer phytochemicals that pose minimal risks to human patients. ART has been shown to arrest cancer cell growth, induce apoptosis, disrupt angiogenic pathways and has other anti-cancer properties through pleiotropic effects as shown against a variety of human cancer cell lines. In addition, ART-related compounds have been shown to inhibit tumor promotion and progression, suggesting these molecules is not only effective as treatment therapeutics, but also as potential anti-cancer preventive agents. ARTs have been recommended and widely used as antimalarials for six years, and they have saved the lives of many patients infected with malaria (WHO, 2006a). Supporting evidence indicates that ART-like compounds may be a therapeutic alternative or adjunct for use in treating highly metastatic and aggressive cancers that have no other long term effective therapy (Morrissey et al., 2010) particularly against cancer cells that have developed drug resistance (Wang et al., 2010). Furthermore, antimalarial endoperoxides may act synergistically with other anti-cancer drugs with no additional side effects. The many antitumor activities, both direct and indirect, of ARTs compounds, however are not entirely explained. So far, the precise molecular events involved in how, when, and where radical oxygen species (ROS) production is initially triggered in cancer cells remain to be defined. In addition, the relevance of any ROS-independent mechanism should be also addressed; these might not be obvious but possibly important for ART-mediated cytotoxicity in some cancer cells. Some other aspects such as the direct DNA damage induced by ART-like compounds and the role of p53 status in genotoxicity need to be further analyzed.
Characterizing the anti-cancer effects of existing and novel ARTs derivatives remains an important research goal, and research also needs to be focused on unveiling the mechanisms of cancer cell cytotoxicity by identifying their relation to particular cancer biomarkers and molecules. ARTs seem to regulate key players participating in multiple pathways such as VEGF, NF-κB, survivin, NOXA, HIF-1α, and BMI-1. These molecules and others are to be revealed, which in turn may be involved in drug response, drug interactions, mechanisms of resistance, and collateral effects in normal cells. A better understanding of common mechanisms under similar conditions in different cell systems will greatly aid the development of targeted ART derivatives. This will improve ARTs cytotoxicity by lowering IC50, emerging of resistance, drug associated toxicity, and potentiating drug interactions. Furthermore, novel endoperoxide compounds and combinational therapies can be addressed to target or co-target markers of carcinoma progression and prevent invasiveness and metastatic properties in highly recurrent and aggressive tumors or advanced stage cancers.
Even though the utility of ARTs in the clinical setting have already been assessed, specific interactions with established chemotherapy regimens need to be further dissected in different cancer cell lines and their associated phenotypes. This will be crucial to implement clinical trials and treatment of individual cases. Due to the toxicity of ARTs, long-term therapy also requires close monitoring. It is important to note that the prototype drug, ART, seems to modulate responses leading to antagonistic interactions with other anti-cancer drugs. While it may be useful to have the prototype drug as a control in vitro, however, its pharmacokinetic properties may differ from the semisynthetic ARTs. Therefore, ART antagonistic reactions and resistance must be cautiously validated using different semisynthetic derivatives. DHA, AS, and AM are the only endoperoxides currently licensed for therapeutic use. So far, AM has been shown to share similar anti-cancer properties as DHA and AS (Wu et al., 2009).
Cancer research is a permanent discovery of new genes and pleiotropic interactions. The study of the antitumor activity of ARTs compounds may become even more complex as immunological hallmarks are also involved in the generation of tumors. Immunological hallmarks in cancer cells include the ability to induce chronic inflammatory response, evasion of tumor recognition, and ability to induce tolerance (Cavallo et al., 2011). Whether ART may participate in the mechanisms involved in these events has yet to be determined. Overall, the real potential and benefits of the ART drug class remain yet to be uncovered. The imminent possibility of ARTs being included in the arsenal of anti-cancer drugs has opened the door for challenging research in this area, one that seems to fulfill many expectations (Crespo-Ortiz and Wei, 2012).
In conclusion, the inhibition of angiogenesis induced by ART-derived drugs has been shown to be a mechanism of anti-cancer activity in vitro and in vivo. In particular, cancer angiogenesis plays a key role in the growth, invasion, and metastasis of tumors. ARTs-induced inhibition of angiogenesis could be a promising therapeutic strategy for treatment and prevention of cancer. Other anti-cancer mechanisms induced by ARTs have been recognized recently that have guided various clinical trials in anti-cancer therapy. Since new and alternative angiogenesis mechanisms have been found, further research on the mechanism of efficacy and toxicity could lead us to understand more deeply the possibilities inherent in therapeutic development of ARTs for malaria, cancer, and other indications. The new therapeutic strategies for use of ARTs as anti-angiogenic agents should be considered to avoid problems associated with reproductive toxicity and neurotoxicity. Taken together, ART and its derivatives have been shown to have potent anti-angiogenic and antivasculogenic effects in tumor cells. These observations have many implications in terms of cancer therapy and prevention as well as avoidance of drug toxicity associated with inhibition of angiogenesis.
Acknowledgments
This material has been reviewed by Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official, or as reflecting the views of the Department of the Army or the Department of Defense.